A) The greater the pressure,the greater the solubility.
B) The greater the pressure,the lower the solubility.
C) You cannot change solubility of a substance by changing the pressure.
D) Nitrogen is not soluble in your blood.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions is the most concentrated?
A) 0.5 L of a 3 molar solution
B) 3.0 L of a 0.5 molar solution
C) 2.0 L of a 1 molar solution
D) 0.5 L of a 1 molar solution
E) 2.0 L of a 2 molar solution
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Many solvents expand to occupy greater volumes with increasing temperature.What happens to the concentration of a solution made with such a solvent as its temperature is increased?
A) Since concentration depends on how much mass is dissolved in a given volume,as the volume increases,the concentration decreases.
B) The concentration of a solution increases as the solute fits into the new spaces between the molecules.
C) Since it has a greater ability to dissolve more solute at a higher temperature,its concentration has decreased.
D) Since it has a greater ability to dissolve more solute at a higher temperature,its concentration has increased.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you filter sea water to remove all of the particles you would be left with a clear
A) homogeneous mixture called a solution.
B) homogeneous mixture called a suspension.
C) heterogeneous mixture called a solution.
D) heterogeneous mixture called a suspension.
E) pure liquid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the solubility of a compound is 30 grams per liter,how much solid is left undissolved if you mix 30 g of the compound in 0.33 L of solution?
A) 20 g
B) 10 g
C) 0 g
D) 30 g
E) 33 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fatty acid molecules can align to form a barrier called a bilipid layer,shown below.In this schematic,the ionic end of the fatty acid is shown as a circle and the nonpolar chain is shown as a squiggly line. 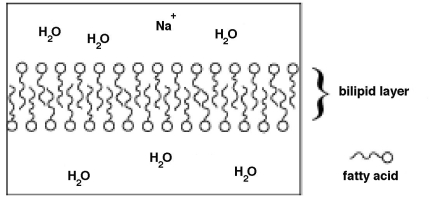 From the schematic above: why do nonpolar molecules have a difficult time passing through the bilipid layer?
From the schematic above: why do nonpolar molecules have a difficult time passing through the bilipid layer?
A) The nonpolar molecules are too large for the space through which they need to pass.
B) The nonpolar molecules are repelled by the polar water molecules.
C) The nonpolar molecules have a difficult time getting past the ionic heads of the fatty acid molecules,which are surrounded by water molecules.
D) Nonpolar molecules tend to bind with the bilipid layer,thus inhibiting their passage.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is the solubility of a gas affected by temperature?
A) As temperature goes up,the solubility goes up.
B) As temperature goes down,the solubility goes down.
C) As temperature goes up,the solubility stays the same.
D) As temperature goes down,the solubility goes up.
E) both A and B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements does not describes the similarity between soaps and detergents?
A) They have a polar ionic end and a long nonpolar tail.
B) Both dissolve oils.
C) Both are synthetic.
D) Both are biodegradable.
E) Both are made from fatty acids.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Account for the observation that ethyl alcohol, OH,dissolves readily in water but dimethyl ether,C OC ,which has the same number and kinds of atoms,does not. 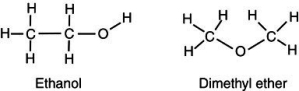
A) The hydrogens on the dimethyl ether surround the molecule,shielding the inner atoms from interacting with the water.
B) Because the carbons arrange themselves in a straight line,the ethanol can interact more easily with more water molecules,thus increasing its solubility.
C) The high electronegativity of the carbon-oxygen-carbon bond on dimethyl ether creates a strong dipole charge on the ends of the molecule,making it highly soluble in water.
D) Because dimethyl ether lacks an -OH group,it is significantly less polar than is ethyl alcohol and is not readily soluble in water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Each circle represents an atom.Which of the following boxes contains an element? A compound? A mixture? 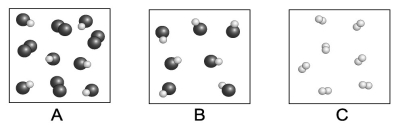
A) element: A,C; compound: A,B,C; mixture: A,B
B) element: C; compound: A,B; mixture: B
C) element: A,C; compound: A,B; mixture: A
D) element: A,C; compound: A,B; mixture: A,B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would cost the least to purify by reverse osmosis?
A) agricultural runoff
B) sea water
C) brackish water
D) All of the above are the same.
E) None of the above can be purified by reverse osmosis.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes a two-molar sucrose solution?
A) one liter of solution that contains 2 moles of sucrose
B) one liter of solution that contains 2 moles of water
C) one liter of solution that contains 6.02 × 1023 molecules of sucrose
D) two liters of solution that contains 1 mole of sucrose
E) one mole of sucrose dissolved in 2 liters of solution
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would you classify the following material? coffee (with milk)
A) a suspension
B) a heterogeneous mixture
C) a solution
D) an element
E) a compound
Correct Answer

verified
Correct Answer
verified
Multiple Choice
During osmosis
A) the water moves into the concentrated solution faster than it leaves.
B) the ions move into the concentrated solution faster than they leave.
C) the water moves more slowly into the concentrated solution than it leaves.
D) the ions move into the concentrated solution slower than they leave.
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Someone argues that he or she doesn't drink tap water because it contains thousands of molecules of some impurity in each glass.How would you respond in defense of the water's purity,if it indeed does contain thousands of molecules of some impurity per glass?
A) Impurities aren't necessarily bad,in fact,they may be good for you.
B) The water contains water molecules and each water molecule is pure.
C) There's no defense.If the water contains impurities it should not be drunk.
D) Compared to the billions and billions of water molecules,a thousand molecules of something else is practically nothing.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does oxygen have such a low solubility in water?
A) Water's attraction for itself is stronger than its attraction for oxygen molecules.
B) Water and oxygen only attract one another by means of weak dipole-induced dipole attractions.
C) The hydrogen bonding in water keeps the oxygen solubility low.
D) Both A and B are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molarity when water is added to 2 moles of sodium chloride to make 0.5 liter of solution?
A) 8 M
B) 4 M
C) 5 M
D) 2.5 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What happens when the molecule-to-molecule attractions in the solute are comparable to those in the solvent?
A) The solute can have infinite solubility in the solvent.
B) The solute does not dissolve in the solvent.
C) The material has only limited solubility in the solvent.
D) The solution will become saturated.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following image represents which kind of matter? 
A) a compound
B) a mixture
C) an element
D) none of the above
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following accurately describes osmosis?
A) The more concentrated solution absorbs water from the less concentrated solution.
B) The less concentrated solution absorbs water from the more concentrated solution.
C) The less concentrated solution gets more dilute.
D) The ions migrate from the more concentrated solution to the less concentrated.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 141
Related Exams