A) Water can act as an acid.
B) Water can act as a base.
C) The conjugate base of water is OH-.
D) The conjugate acid of water is H3O+.
E) All of the above are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of H+ in a solution with pH = 2.50?
A) 4.5 M
B) 1.3 M
C) 0.0032 M
D) 0.40 M
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The burning of fossil fuels produces nitrogen oxides and sulfur oxides which can react to form acid rain (nitric and sulfuric acids).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution at 25°C has a hydrogen ion concentration of 2.6 × 10-5 M.Which of the following is TRUE?
A) [H3O+] > [OH-]
B) [H3O+] < [OH-]
C) [H3O+] = [OH-]
D) [H3O+] = ![]()
E) none of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following statements are TRUE of buffer solutions? 1.A buffer solution can be made by mixing equal concentrations of Acetic acid and sodium acetate. 2.A buffer solution can be made by mixing equal concentrations of Hydrochloric acid and sodium chloride. 3.A buffer solution resists changes in pH when small quantities of acid Or base are added.
A) 1 and 2 only
B) 2 and 3 only
C) 1 and 3 only
D) All of 1,2,and 3
E) None of 1,2,and 3
Correct Answer

verified
Correct Answer
verified
True/False
The products of a neutralization reaction are carbon dioxide and water.
Correct Answer

verified
Correct Answer
verified
True/False
Bases feel slippery.
Correct Answer

verified
True
Correct Answer
verified
True/False
In general,the stronger the acid,the weaker is its conjugate base.
Correct Answer

verified
Correct Answer
verified
True/False
A strong acid must also be a strong electrolyte.
Correct Answer

verified
Correct Answer
verified
True/False
The ion product constant for water (Kw)is best stated as Kw = [H2O] [H+].
Correct Answer

verified
Correct Answer
verified
True/False
A solution with a concentration of 0.000 000 1 M HCl would be an acidic solution.
Correct Answer

verified
Correct Answer
verified
True/False
Bases have a bitter taste.
Correct Answer

verified
Correct Answer
verified
True/False
The conjugate base to HSO4- is SO42-.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The titration of 25.00 mL a 0.125 M HClO4 solution required 21.37 mL of KOH to reach the endpoint.What is the concentration of the KOH?
A) 0.292 M
B) 0.0668 M
C) 0.146 M
D) 0.134 M
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT an acid-base conjugate pair?
A) H2CO3 and HCO3-
B) H2O and OH-
C) H2S and OH-
D) NH4+ and NH3
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Water always acts as an acid in reactions.
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
The Arrhenius definition of an acid is:
A) a proton donor.
B) a proton acceptor.
C) produces H⁺ in solution.
D) produces OH⁻ in solution.
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Bases feel slippery because they react with oils on your skin to form soap-like substances.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of the hydroxide ion given that the concentration of the hydronium ion is 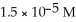 ?
?
A) 6.7 × ![]() M
M
B) 1.5 ![]() M
M
C) 1.0 ![]() M
M
D) 1.0 ![]() M
M
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carboxylic acids can be found in:
A) grapes.
B) apples.
C) lemons.
D) all of the above
E) none of these
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 117
Related Exams