A) 0°C
B) 38°C
C) 95°C
D) 273°C
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Gases and liquids are compressible,but solids are not.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following diatomic elements would have a mass of 19.08 grams stored in a 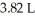 container at
container at 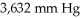 and 100°C? (R= 0.0821 L atm/ mol K)
and 100°C? (R= 0.0821 L atm/ mol K)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) not enough information.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major component of the air we breathe?
A) nitrogen
B) oxygen
C) argon
D) carbon dioxide
E) smog
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE for gases? 1.The temperature of a gas is inversely proportional to its pressure. 2.The volume of a gas is directly proportional to the pressure in torr. 3.The pressure of a gas is due to collisions of the gas molecules.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2 only
E) 1 and 3 only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the volume (in liters) of 1.00 mole of krypton gas that has a pressure of 1140 mm Hg and a temperature of 25.0°C? (R= 0.0821 L atm/ mol K)
A) 1.37
B) 16.3
C) 0.0215
D) 0.00180
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Absolute zero refers to 0°C.
Correct Answer

verified
Correct Answer
verified
True/False
Aerosol spray cans contain gas trapped in a fixed volume and cans of this type can explode if heated to high temperature.This illustrates that pressure and temperature are directly proportional.
Correct Answer

verified
Correct Answer
verified
True/False
A sealed bag of potato chips will expand when taken to a higher altitude.This is an example of Boyle's law.
Correct Answer

verified
Correct Answer
verified
True/False
Gas particles act independently of each other.
Correct Answer

verified
Correct Answer
verified
True/False
If you had a five liter balloon of argon gas and a five liter balloon of xenon gas,and you removed 10 grams of gas from each balloon,the balloons would both shrink down to the same size.
Correct Answer

verified
Correct Answer
verified
True/False
The vapor pressure of water is independent of temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 3.76 g sample of a noble gas is stored in a 2.00 L vessel at 874 torr and 25°C.What is the noble gas? (R= 0.0821 L atm/ mol K)
A) He
B) Ne
C) Ar
D) Kr
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
To solve problems using Avogadro's Law,which mathematical equation should be used?
A) ![]() =
= ![]()
B) ![]()
![]() =
= ![]()
![]()
C) ![]() =
= ![]()
D) ![]()
![]() =
= ![]()
![]()
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the final volume of a balloon that was initially 500.0 mL at 25°C and was then heated to 50°C?
A) 461 mL
B) 193 mL
C) 1.00 L
D) 542 mL
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A pedometer is a device created by Torricelli to measure pressure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
1 torr is equal to:
A) 760 mm Hg.
B) 1 mm Hg.
C) 1 Pa.
D) 14.7 psi.
E) all of the above
Correct Answer

verified
Correct Answer
verified
True/False
The volume of 9.00 grams of water vapor at STP is 11.2 L.
Correct Answer

verified
Correct Answer
verified
True/False
If the column of mercury in a barometer drops to a lower reading,this means the measured pressure has decreased.
Correct Answer

verified
Correct Answer
verified
True/False
The unit of pressure known as the atmosphere (atm)is defined as the average pressure found at the top of Mount Everest.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 123
Related Exams