A) Ca(NO3) 2
B) Li3PO4
C) LiNO3
D) Ca3(PO4) 2
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
The limiting reactant determines what the actual yield is.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of water are made from complete reaction of 1.4 moles of hydrogen gas? Given the reaction: 2H2 + O2 → 2H2O
A) 2.8
B) 0.7
C) 1.4
D) 2.1
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of lithium nitrate are theoretically produced if we start with 3.4 moles of Ca(NO3) 2 and 2.4 moles of Li3PO4? Reaction: 3Ca(NO3) 2 + 2Li3PO4 → 6LiNO3 + Ca3(PO4) 2
A) 7.2
B) 6.8
C) 1.2
D) 1.1
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
Given the recipe: 2 cups flour + 1 egg + 3 oz blueberries → 4 muffins If you have 9 cups of flour,4 eggs and plenty of blueberries,the theoretical yield of muffins is 16.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider a reaction of chemicals depicted as A + B → C + D.Suppose reaction of 17.8 grams of A with 29.5 grams of B gave 24.7 grams of C.However,the theoretical yield of C for this reaction was 33.3 grams.What was the percent yield of chemical C?
A) 74.2
B) 47.3
C) 11.7
D) 135
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The conversion factor between mass and moles for a compound is the molar mass.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: 2 Al + 3Br2 → 2 AlBr3 Suppose a reaction vessel initially contains 5.0 mole Al and 6.0 mole Br2.What is in the reaction vessel once the reaction has occurred to the fullest extent possible?
A) 1.0 mole Al; 0 mole Br2; 4.0 mole AlBr3
B) 0 mole Al; 0 mole Br2; 11.0 mole AlBr3
C) 2.0 mole Al; 3.0 mole Br2; 2.0 mole AlBr3
D) 0 mole Al; 1.0 mole Br2; 4.0 mole AlBr3
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Given the recipe: 2 cups flour + 1 egg + 3 oz blueberries → 4 muffins. You can make 9 muffins from 3.5 cups of flour.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction for the oxidation of NH3 is given as: 4 NH3 + 5 O2 → 4 NO + 6 H2O Under certain conditions the reaction will proceed at 29.8% yield of NO.How many grams of NH3 must be made to react with excess oxygen to yield 70.5 g of NO?
A) 134
B) 237
C) 21.0
D) 2.37
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the excess reactant for the following reaction given we have 2.6 moles of HCl and 1.4 moles of Ca(OH) 2? Reaction: 2HCl + Ca(OH) 2 → 2H2O + CaCl2
A) Ca(OH) 2
B) HCl
C) H2O
D) CaCl2
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
The percent yield is calculated by dividing the actual yield by the theoretical yield times 100.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of aluminum oxide are produced according to the reaction below given that you start with 10.0 grams of Al and 19.0 grams of O2? Reaction: 4Al + 3O2 → 2Al2O3
A) 40.4
B) 5.00
C) 0.185
D) 18.9
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
Given the reaction: 2 Na(s)+ Cl2(g)→ 2 NaCl(s) The conversion factor for chlorine gas to sodium chloride is: 1 mol Cl2 ≡ 2 mol NaCl
Correct Answer

verified
Correct Answer
verified
True/False
Given the reaction: 2 Na(s)+ Cl2(g)→ 2 NaCl(s) The conversion factor for chlorine gas to sodium metal is: 2 mol Cl ≡ 2 mol Na
Correct Answer

verified
Correct Answer
verified
True/False
The limiting reactant is not necessarily the reactant with the least mass.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the percent yield of CuS for the following reaction given that you start with 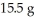 of Na2S and
of Na2S and 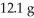 CuSO4?
The actual amount of CuS produced was
CuSO4?
The actual amount of CuS produced was 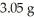 . Reaction: Na2S + CuSO4 → Na2SO4 + CuS
. Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) 16.1%
B) 42.1%
C) 18.93%
D) 7.25%
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
The limiting reactant is the product that is completely consumed in a chemical reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ingredient is the limiting reactant if you have 6 cups of flour,9 eggs and 2 tbs of oil? Given: 2 cups flour + 3 eggs + 1 tbs oil → 4 waffles
A) flour
B) eggs
C) oil
D) waffles
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of water are made from the reaction of 4.0 grams of hydrogen gas? Given the reaction: 2H2 + O2 → 2H2O
A) 18
B) 72
C) 36
D) 4.5
E) not enough information
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 115
Related Exams