A) ![]() Th
Th
B) ![]() Np
Np
C) ![]() Ac
Ac
D) ![]() U
U
E) ![]() Th
Th
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If we start with 1.000 g of cobalt-60,0.675 g will remain after 3.00 yr.This means that the half-life of cobalt-60 is ________ yr.
A) 3.08
B) 4.44
C) 2.03
D) 5.30
E) 7.65
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents what nuclear process?
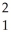 H +
H +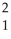 H →
H →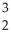 He +
He +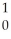 N
N
A) nuclear fusion
B) alpha emission
C) beta emission
D) nuclear fission
E) neutron capture
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the mass defect in Fe-56 if the mass of an Fe-56 nucleus is 55.921 amu.The mass of a proton is 1.00728 amu and the mass of a neutron is 1.008665 amu.
A) 0.528 amu
B) 3.507 amu
C) 0.564 amu
D) 1.056 amu
E) 0.079 amu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If we start with 1.000 g of strontium-90,0.908 g will remain after 4.00 yr.This means that the half-life of strontium-90 is ________ yr.
A) 3.05
B) 4.40
C) 28.8
D) 3.63
E) 41.6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements would be expected to be particularly stable?
A) ![]() C
C
B) ![]() B
B
C) ![]() Be
Be
D) ![]() Li
Li
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the element that is not used as a radioactive tracer.
A) iron-59
B) phosphorus-32
C) thallium-201
D) iodine-131
E) carbon-13
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which nuclide below is most likely to decay by electron capture?
A) ![]() Re
Re
B) ![]() Re
Re
C) ![]() Re
Re
D) ![]() Re
Re
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -beta particle
A) ![]() γ
γ
B) ![]() p
p
C) ![]() n
n
D) ![]() e
e
E) ![]() He
He
F) ![]() e
e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write a nuclear equation to describe the neutron induced fission of U-235 to form Xe-134 and Sr-100.Determine how many neutrons are produced in the reaction.
A) 4
B) 3
C) 1
D) 0
E) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the symptom that is not from radiation exposure.
A) measles
B) increased cancer risk
C) death
D) genetic effects
E) weaker immune systems
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A geological sample is found to have a Pb-206/U-238 mass ratio of 0.337/1.00.Assuming there was no Pb-206 present when the sample was formed,how old is it? The half-life of U-238 is 4.5 × 109 years.
A) 7.3 × 1011 years
B) 1.4 × 1010 years
C) 2.4 × 1010 years
D) 2.1 × 109 years
E) 7.1 × 109 years
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon-11 is used in medical imaging.The half-life of this radioisotope is 20.4 min.What percentage of a sample remains after 60.0 min?
A) 71.2
B) 5.28
C) 13.0
D) 34.0
E) 2.94
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which particle has the highest penetrating power?
A) alpha particle
B) beta particle
C) gamma particle
D) positron emission
E) electron capture
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents what nuclear process?
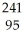 Am →
Am →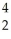 He +
He +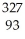 Np
Np
A) beta emission
B) neutron bombardment
C) alpha emission
D) electron capture
E) positron emission
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the beta decay of 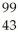 Tc.
Tc.
A) ![]() Ru
Ru
B) ![]() Rh
Rh
C) ![]() Nb
Nb
D) ![]() Mo
Mo
E) ![]() Ru
Ru
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the positron emission of 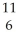 C.
C.
A) ![]() B
B
B) ![]() N
N
C) ![]() C
C
D) ![]() B
B
E) ![]() N
N
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Stable isotopes,with low atomic numbers,have a N/Z ratio of 1.What does that imply?
A) The number of neutrons equals the number of protons.
B) The number of neutrons equals the number of electrons plus protons.
C) The number of protons equals the number of electrons.
D) The atomic number equals the atomic mass.
E) The number of protons equals the number of electrons plus neutrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the elements used in radiometric dating.
A) Carbon-14 to nitrogen-14.
B) Uranium-238 to lead-206.
C) Potassium-40 to argon-40.
D) None of the above.
E) All of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Neptunium-239 has a half-life of 2.35 days.How many days must elapse for a sample of 239Np to decay to 0.100% of its original quantity?
A) 0.0427 days
B) 1.491 days
C) 2.04 days
D) 23.4 days
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 105
Related Exams