A) 72.9 atm, 31 °C
B) 5.1 atm, -56.7 °C
C) 1 atm, -78.5 °C
D) 5.1 atm, 31 °C
E) 72.9 atm, -56.7 °C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the strongest type of intermolecular force present in CH2F2?
A) dispersion
B) dipole-dipole
C) hydrogen bonding
D) ion-dipole
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the substance with the highest vapor pressure at a given temperature.
A) SiS2
B) RbCl
C) CH3SCH3
D) BF3
E) SbH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the term used to describe the ability of a liquid to flow against gravity up a narrow tube.
A) capillary action
B) viscosity
C) surface tension
D) density
E) none of the above
Correct Answer

verified
Correct Answer
verified
Essay
Define boiling point of a liquid.
Correct Answer

verified
The temperature at w...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Choose the molecule or compound that exhibits dispersion forces as its strongest intermolecular force.
A) O2
B) CO
C) HF
D) NaCl
E) All of these have intermolecular forces stronger than dispersion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the place which has the lowest boiling point of water.
A) Death Valley, 282 feet below sea level
B) a pressurized passenger jet, 35,000 feet
C) New Orleans, sea level
D) Mt. Everest, 29,035 feet
E) Denver, Colorado, 5280 feet
Correct Answer

verified
Correct Answer
verified
Short Answer
Define volatile.
Correct Answer

verified
Liquids th...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Based on the figure above,the boiling point of ethyl alcohol under an external pressure of 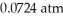 is ________ °C.
is ________ °C.
A) 80
B) 60
C) 70
D) 40
E) 20
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the characteristics of a gas.
A) indefinite shape and volume
B) indefinite shape, but definite volume
C) definite shape and volume
D) none of the above
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the phase diagram shown.What is the normal boiling point? 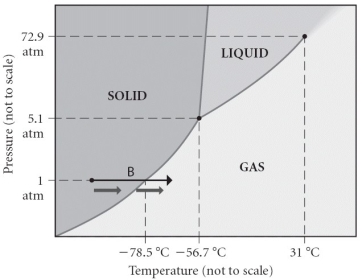
A) 31°C
B) -56.7 °C
C) -78.5 °C
D) 0°C
E) 100°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much energy is required to vaporize 158 g of butane (C4H10) at its boiling point,if its ΔHvap is 24.3 kJ/mol?
A) 15.1 kJ
B) 66.1 kJ
C) 41.9 kJ
D) 2.60 kJ
E) 38.4 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following compounds in order of increasing strength of intermolecular forces. CH4 CH3CH2CH3 CH3CH3
A) CH3CH2CH3 < CH4 < CH3CH3
B) CH3CH2CH3 < CH3CH3 < CH4
C) CH3CH3 < CH4 < CH3CH2CH3
D) CH4 < CH3CH2CH3 < CH3CH3
E) CH4 < CH3CH3 < CH3CH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heat of vaporization of acetone is 31.3 kJ/mol,and a a boiling point of 78.6° at 1520 mm Hg.What is the boiling point when the pressure is raised to 7600 mm Hg?
A) 211°C
B) 182°C
C) 83.5°C
D) 141°C
E) 118°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the substance with the highest surface tension.
A) HOCH2CH2OH
B) CH2Br2
C) CH3CH2Cl
D) CH3CH2OH
E) CH3CH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds exhibits hydrogen bonding?
A) CH3I
B) HBr
C) CH3OCH3
D) CH3NH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Define freezing.
A) the phase transition from solid to gas
B) the phase transition from gas to solid
C) the phase transition from gas to liquid
D) the phase transition from liquid to gas
E) the phase transition from liquid to solid
Correct Answer

verified
Correct Answer
verified
Essay
Why do O,F and N,when bonded to H,form such strong intermolecular attractions to neighboring molecules? Make sure to be specific.
Correct Answer

verified
Oxygen,fluorine and nitrogen are all ver...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
In a liquid,the energy required to increase the surface area by a unit amount is called
A) viscosity.
B) surface tension.
C) dipole-dipole force.
D) hydrogen bonding.
E) capillary action.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Define boiling.
A) A liquid becomes a gas.
B) A gas becomes a liquid.
C) A gas becomes a solid.
D) A solid becomes a gas.
E) A solid becomes a liquid.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 132
Related Exams