A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
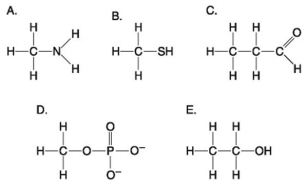 -Which molecule shown above contains a functional group that cells use to transfer energy between organic molecules?
-Which molecule shown above contains a functional group that cells use to transfer energy between organic molecules?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating the differences between three organic compounds. They all have the same carbon skeleton but have differences in their functional groups. -Organic chemistry is currently defined as
A) the study of compounds made only by living cells.
B) the study of carbon compounds.
C) the study of vital forces.
D) the study of natural (as opposed to synthetic) compounds.
E) the study of hydrocarbons.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Stanley Miller's 1953 experiments assumed that early Earth's atmosphere contained
A) hydrogen cyanide, formaldehyde, hydrogen gas, and water vapour.
B) ammonia, methane, hydrogen gas, and water vapour.
C) ammonia, methane, oxygen gas, and water vapour.
D) amino acids, methane, hydrogen cyanide, and water vapour.
E) methane, formaldehyde, ammonia, and carbon dioxide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a false statement concerning amino groups?
A) They are basic in pH.
B) They are found in amino acids.
C) They contain nitrogen.
D) They are nonpolar.
E) They are components of urea.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
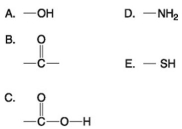 -Which of the groups above is an acidic functional group that can dissociate and release H⁺ into a solution?
-Which of the groups above is an acidic functional group that can dissociate and release H⁺ into a solution?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element present in all organic molecules is
A) hydrogen.
B) oxygen.
C) carbon.
D) nitrogen.
E) phosphorus.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Differences among organisms are caused by
A) large differences in elemental composition from organism to organism.
B) differences in the types and relative amounts of organic molecules synthesized by each organism.
C) differences in the elements that bond with carbon in each organism.
D) differences in the sizes of the organic molecules in each organism.
E) differences in inorganic compounds present in each organism.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, a hydrocarbon chain with the same number of carbon atoms, but with one or more double bonds, will
A) be more flexible in structure.
B) be more constrained in structure.
C) be more polar.
D) have more hydrogen atoms.
E) have fewer structurally distinct isomers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complexity and variety of organic molecules is due to
A) the chemical versatility of carbon atoms.
B) the variety of rare elements in organic molecules.
C) the fact that they can be synthesized only in living organisms.
D) their interaction with water.
E) their tremendously large sizes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Which of the groups shown above is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?
-Which of the groups shown above is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three or four of the following illustrations depict different structural isomers of the organic compound with molecular formula C₆H₁₄. For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted. Which one, if any, is NOT a structural isomer of this compound?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) Each of the illustrations in the other answer choices depicts a structural isomer of the compound with molecular formula C₆H₁₄.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon atom is most likely to form what kind of bond(s) with other atoms?
A) ionic
B) hydrogen
C) covalent
D) covalent bonds and hydrogen bonds
E) ionic bonds, covalent bonds, and hydrogen bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Stanley Miller's 1953 experiments proved that
A) life arose on Earth from simple inorganic molecules.
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth.
C) life arose on Earth from simple organic molecules, with energy from lightning and volcanoes.
D) the conditions on early Earth were conducive to the origin of life.
E) the conditions on early Earth were conducive to the abiotic synthesis of organic molecules.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the biologically important functional groups listed below, which is not reactive but serves as a tag or marker?
A) hydroxyl
B) carboxyl
C) amino
D) sulfhydryl
E) methyl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating the differences between three organic compounds. They all have the same carbon skeleton but have differences in their functional groups. -Which of the following hydrocarbons has a double bond in its carbon skeleton?
A) C₃H₈
B) C₂H₆
C) CH₄
D) C₂H₄
E) C₂H₂
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Which of the groups shown above is a carbonyl functional group?
-Which of the groups shown above is a carbonyl functional group?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating the differences between three organic compounds. They all have the same carbon skeleton but have differences in their functional groups. -The third molecule displays both acidic and basic properties. It most likely has
A) at least one carboxyl group.
B) at least one amino group.
C) at least one hydroxyl group.
D) both an amino and a carboxyl group.
E) both an hydroxyl and a carboxyl group.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Amino acids are acids because they always possess which functional group?
A) amino
B) carbonyl
C) carboxyl
D) phosphate
E) hydroxyl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of molecules shown below are not enantiomers of a single molecule?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 78
Related Exams