Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species has the highest standard entropy (S°) for one mole of substance?
A) Au(s)
B) Cd(s)
C) Hg(l)
D) Ni(s)
E) K(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the data: C2H4(g) , H°f = +51.9 kJ mol-1, S° = 219.8 J mol-1 K-1 CO2(g) , H°f = -394 kJ mol-1, S° = 213.6 J mol+1 K-1 H2O(l) , H°f = -286.0 kJ mol-1, S° = 69.96 J mol-1 K-1 O2(g) , H°f = 0.00 kJ mol-1, S° = 205 J mol-1 K-1 Calculate the maximum amount of work that can be obtained, at 25.0 °C, from the process:C2H4(g) + 3 O2(g) 2 CO2(g) + 2 H2O(l)
A) 1332 kJ mol-1
B) 1380 kJ mol-1
C) 1451 kJ mol-1
D) 1492 kJ mol-1
E) 2422 kJ mol-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which set below has the species listed in order of increasing standard entropy, S°?
A) CaSO4(s) < C2H5OH(l) < Ar(g)
B) CH3CH2-O-H(l) < Ar(g) < CaSO4(s)
C) CaSO4(s) < Ar(g) < C2H5OH(l)
D) C2H5OH(l) < CaSO4(s) < Ar(g)
Correct Answer

verified
Correct Answer
verified
True/False
A negative value for the standard molar entropy, S°, of a chemical substance indicates that the substance is unstable and may decompose or detonate if mishandled.
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider a reaction that is both endothermic and increasingly ordered. How will temperature affect the spontaneity of the process?
Correct Answer

verified
We need to utilize the equation ΔG = ΔH ...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
For the system, 2 NO2(g)  N2O4(g), ΔG° = −5.40 kJ. Calculate the value of the equilibrium constant, Kp, for this system.
N2O4(g), ΔG° = −5.40 kJ. Calculate the value of the equilibrium constant, Kp, for this system.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the best example of a thermodynamically reversible process?
A) water dripping out of a leak in a pail
B) the conversion of ice to water at 0°C
C) the popping of a balloon
D) the dissolving of sugar into water
E) the cracking of a block of ice with a hammer
Correct Answer

verified
Correct Answer
verified
Short Answer
For the reaction H2(g)+ S(s)→ H2S(g), ΔH° = -20.2 kJ/mol and ΔS° = +43.1 J/K·mol, at which temperatures would the reaction be spontaneous?
Correct Answer

verified
For all te...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which set has the species listed in order of increasing standard entropy, S°?
A) Au(s) < CaCO3(s) < H2O(l)
B) CaCO3(s) < H2O(l) < Au(s)
C) Au(s) < H2O(l) < CaCO3(s)
D) CaCO3(s) < Au(s) < H2O(l)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider a reaction that is both endothermic and increases entropy. How will temperature affect the spontaneity of the process?
Correct Answer

verified
We need to utilize the equation ΔG = ΔH ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which species has the greatest standard entropy (S°) for one mole of substance?
A) I2(s)
B) Br2(l)
C) N2(l)
D) Cl2(g)
E) He(l)
Correct Answer

verified
Correct Answer
verified
Short Answer
Using the data,
I2(g), ΔH°f = +62.4 kJ mol−1, S° = +260.7 J mol−1 K−1
I2(s), ΔH°f = 0.00 kJ mol−1, S° = +116.12 J mol−1 K−1
calculate the temperature at which solid iodine should have a vapor pressure of 1.00 atm, based on the reaction, I2(g)  I2(s).
I2(s).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The normal melting point of benzoic acid is 122.4°C. Predict the signs of H, S, and ?G for the process in which liquid benzoic acid freezes at 120°C and 1 atm: C7H6O2(l) .C7H6O2(s) 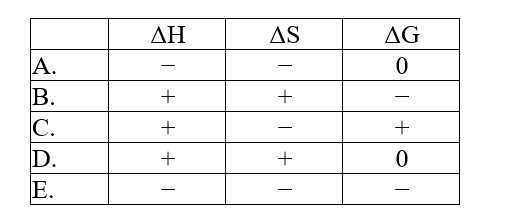
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which process is accompanied by an increase in the entropy of the system?
A) solid gold melting
B) the condensation of water on cold surface
C) the freezing of a popsicle
D) sewing a quilt
E) a cup of coffee cooling in a mug
Correct Answer

verified
Correct Answer
verified
True/False
The standard entropy, S° for an element in its standard state is always zero.
Correct Answer

verified
Correct Answer
verified
Essay
Describe, using the free energy relationship ΔG = ΔH - TΔS, the process of a hot object coming into thermal equilibrium with a cold object.
Correct Answer

verified
Initially the hot object has a large H b...View Answer
Show Answer
Correct Answer
verified
View Answer
True/False
Three factors which, acting together, determine whether a reaction is spontaneous or not, are entropy, internal energy, and enthalpy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant at 25°C for the reaction 2NO(g) + O2(g)  2NO2(g) is Kp = 1.65 × 1012. Calculate the value of G° for the reaction at this temperature.
2NO2(g) is Kp = 1.65 × 1012. Calculate the value of G° for the reaction at this temperature.
A) -4.09 kJ/mol
B) -5.85 kJ/mol
C) +5.85 kJ/mol
D) -69.7 kJ/mol
E) 1.65 kJ/mol
Correct Answer

verified
Correct Answer
verified
Short Answer
The normal freezing point of ammonia is -78°C. Predict the signs of ΔH, ΔS, and ΔG for ammonia when it melts at -76°C and 1 atm: NH3(s)→ NH3(l)
Correct Answer

verified
Melting is an endothermic process, so ΔH...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 41 - 60 of 109
Related Exams