A) 346 km/s away from the observer
B) 346 km/s toward the observer
C) 1.3 × 108 m/s away from the observer
D) 1.3 × 108 m/s toward the observer
E) The radial velocity of the star cannot be determined from this information.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Diagram 6-1 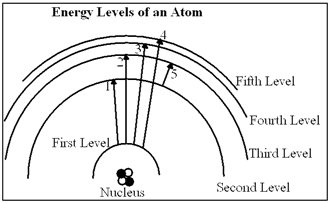 In Diagram 6-1, which of the transitions would absorb a photon with the smallest energy?
In Diagram 6-1, which of the transitions would absorb a photon with the smallest energy?
A) Transition 1
B) Transition 2
C) Transition 3
D) Transition 4
E) Transition 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
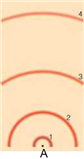 In the above model of an atom, where is the neutron located, assuming a neutron is present?
In the above model of an atom, where is the neutron located, assuming a neutron is present?
A) A
B) 1
C) 2
D) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The neutral carbon atom consists of
A) one proton and one neutron.
B) six protons.
C) one proton, one neutron, and one electron.
D) six protons and six electrons.
E) an isotope and an ion.
Correct Answer

verified
Correct Answer
verified
Short Answer
A zero Kelvin temperature is also known as ________________________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One star has a temperature of 30,000 K and another star has a temperature of 6,000 K. Compared to the cooler star, how much more energy per second will the hotter star radiate from each square meter of its surface?
A) 5 times
B) 25 times
C) 8.1 × 1017 times
D) 625 times
E) 1.3 × 1015 times
Correct Answer

verified
Correct Answer
verified
Short Answer
Electrons in an atom are attracted to the nucleus by the _______________ force.
Correct Answer

verified
Correct Answer
verified
True/False
A hotter star is brighter than a cooler star of the same size. Assume both are typical stars.
Correct Answer

verified
Correct Answer
verified
True/False
An atom gained an electron. Therefore it is now called an ion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a fundamental component of the atom?
A) proton
B) neutron
C) ion
D) electron
Correct Answer

verified
Correct Answer
verified
True/False
Isotopes of the same element have the same number of protons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
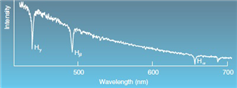 Which series is shown above?
Which series is shown above?
A) Lyman
B) Paschen
C) Balmer
D) None of the other choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The neutral hydrogen atom consists of
A) one proton and one neutron.
B) one proton.
C) one proton, one neutron, and one electron.
D) one proton and one electron.
E) an isotope and an ion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Since an electron is so less massive than a proton, what is the approximate mass and charge of a neutral hydrogen atom?
A) the mass of an electron, negative
B) the mass of a proton, neutral
C) the mass of a neutron, positive
D) the mass of a proton and neutron, positive
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The H δ line has a wavelength of 410.2 nm when observed in the laboratory. If the H δ line appears in a stars spectrum at 410.0 nm, what is the radial velocity of the star?
A) 146 km/s away from the observer
B) 146 km/s toward the observer
C) 6.0 × 107 m/s away from the observer
D) 6.0 × 107 m/s toward the observer
E) The radial velocity of the star cannot be determined from this information.
Correct Answer

verified
Correct Answer
verified
True/False
The Lyman series lines of hydrogen all lie in the infrared.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A(n) ____ contains two or more atoms that are bound together by exchanging or sharing electrons with each other.
A) nucleus
B) ion
C) proton
D) electron cloud
E) molecule
Correct Answer

verified
Correct Answer
verified
Short Answer
When the electrons in an atom are in their lowest possible energy levels, the atom is said to be in its ____________________ state.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
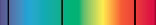 To create the above spectra, which of the following is NOT true?
To create the above spectra, which of the following is NOT true?
A) A dense gas is excited and the light produced is over all wavelengths.
B) A low-density gas is excited and the light produced is over discrete wavelengths.
C) Light from a low-density gas passes through a warm, dense gas and the light that is produced is at all wavelengths except for a few discrete wavelengths.
D) All of the other choices are not true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most massive part of the atom is(are) the ____ which has(have) a ____ charge.
A) electrons; negative
B) nucleus; negative
C) electrons; positive
D) nucleus; positive
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 125
Related Exams