A) Alpha emission
B) Beta emission
C) Radioactive decay
D) Nuclear fission
E) Nuclear fusion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of Nitrogen - 16 is 7 seconds. How long does it take for 100 mg of Nitrogen - 16 to be reduced tO6.25 mg?
A) 1.75 seconds
B) 4 seconds
C) 5 seconds
D) 28 seconds
E) 35 seconds
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What is the missing particle in the nuclear equation?
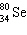 + ____
+ ____ 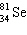 +
+ 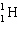
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosphorous - 32 has a half-life of 14.3 days. How many days are required for 4.00 grams of phosphorous - 32 to decay to 1.00 gram?
A) 1.80 days
B) 16.1 days
C) 28.6 days
D) 42.9 days
E) 57.2 days
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass of an 1H atom is 1.007825 u, and a neutron has a mass of 1.008665 u. The atomic mass of 23Na is 22.9898 u. What is the mass defect of this nuclide in u?
A) 0.2003 u
B) 0.1994 u
C) 0.1901 u
D) 0.1892 u
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of parchment produces 10.3 disintegrations of 14C per minute per gram of carbon. Estimate the age of the parchment, assuming the original activity was 15.3 disintegrations per minute per gram of carbon. (t1/2 = 5730 years)
A) 8.5×103 yr
B) 3.9×103 yr
C) 3.3×103 yr
D) 2.3×103 yr
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following components did Rutherford identify in radioactive decay?
A) "42 a particles"
B) "0 - 1 b particles"
C) "00 g rays"
D) "answer a and b"
E) "all of these"
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider comparing the two Phosphorous radionuclides given below and their corresponding half-life periods. Which statement below is true? Isotope Half-life period Phosphorous - 28 0.28 seconds Phosphorous - 32 14.3 days
A) Phosphorous - 28 decays faster and is the most stable nuclide.
B) Phosphorous - 28 decays faster and is the least stable nuclide.
C) Phosphorous - 32 decays faster and is the most stable nuclide.
D) Phosphorous - 32 decays faster and is the least stable nuclide.
E) They both decay at the same rate but Phosphorous - 28 is more stable.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What must be placed in the blank to produce a balanced nuclear equation?
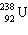 +
+ 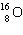 ____ +
____ + 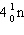
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following statements: I. Control rods are used in a nuclear reactor to adjust the power and prevent the reaction from becoming supercritical. II. Control rods in a nuclear reactor are made of a material that absorbs neutrons. III. To obtain energy from a nuclear reactor, the fission reaction must be supercritical. Which of the following choices is correct?
A) only I is true
B) only II is true
C) I and III are true, II is false
D) I and II are true, III is false
E) I, II, and III are all true
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The binding energy per nucleon is greatest for:
A) the very light atoms.
B) the very heavy atoms.
C) atoms with odd numbers of both neutrons and protons.
D) the stable isotopes of elements near iron.
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a nuclear reaction, the nuclide 75As absorbs a neutron and emits a gamma ray. The product nucleus is:
A) "76Ge."
B) "76Se."
C) "72Ga."
D) "76As."
E) "none of these"
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample that contains 5.7×10 - 9 mol of 93Zr decays at a rate of 3.02×103 disintegrations per minute. What is the half-life of 93Zr? (1 year = 5.256×105 minutes)
A) 1.0×106 years
B) 1.5×106 years
C) 2.2×106 years
D) The half-life cannot be found from this information.
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a nuclide with atomic number = Z and mass number = A undergoes 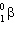 decay, the product nucleus will have:
decay, the product nucleus will have:
A) atomic number = Z, mass number = A
B) atomic number = Z - 2, mass number = A - 4.
C) atomic number = Z + 1, mass number = A.
D) atomic number = Z - 1, mass number = A.
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which form of radioactive decay can penetrate skin? I. Gamma radiation II. Beta particles III. Alpha particles
A) I only
B) II only
C) III only
D) I and II
E) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The type of radioactivity that removes excess energy from the nucleus without changing the atomic number or mass number is called:
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The alpha decay of 231Pa produces:
A) "227Ac."
B) "229Fr."
C) "227Np."
D) "229U."
E) "none of these"
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of nuclear reaction is illustrated below? 23592U + 10n→13954Xe + 9538Sr + 2 10n
A) Alpha emission
B) Beta emission
C) Radioactive decay
D) Nuclear fission
E) Nuclear fusion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Polonium - 218 undergoes alpha emission. What is the daughter nuclide after polonium - 218 decays?

A) "21486Rn"
B) "22288Ra"
C) "21482Pb"
D) "21885At"
E) "21883Bi"
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What daughter nuclide results after carbon - 14 undergoes beta decay ?
A) "147N"
B) "145B"
C) "156C"
D) "104Be"
E) "136C"
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 89
Related Exams