A) A sodium atom and a chlorine atom share two electrons.
B) A sodium atom loses an electron and the chlorine atom accepts the electron.
C) A chlorine atom loses an electron and the sodium atom accepts the electron.
D) A sodium atom loses an electron and the chlorine atom loses seven electrons.
E) A sodium atom gains seven electrons and the chlorine atom loses seven electrons.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the three Lewis structures listed below, pick the best answer.
I. 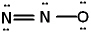 II.
II.  III.
III. 
A) I and II are correct, III is incorrect
B) II and III are correct, I is incorrect
C) I and III are correct, II is incorrect
D) all three are correct
E) only II is correct
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis structure below obeys the octet rule for every atom in the structure of SF2 and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis structure below obeys the octet rule for every atom in the structure of SO3 and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species listed below has the largest lattice energy?
A) CaCl2
B) LiCl
C) MgF2
D) NaCl
E) SrBr2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a false statement regarding the differences between ionic and covalent bonding?
A) Ionic bonds form from sharing electrons and covalent bonds form by a complete transfer of electrons from one atom to another.
B) The driving force for the formation of both ionic and molecular compounds is to attain a stable "Noble Gas" electronic configuration.
C) Ionic bonds form between a metal and a nonmetal whereas covalent bonds form between two nonmetals.
D) Ionic compounds exist as extended arrays of alternating cations and anions whereas covalently bonded compounds exist as discrete entities with weak forces of interaction among the molecules.
E) They are all true statements.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecule N2H4 (H2NNH2 connectivity) has in its correct Lewis structure a total of:
A) 4 bonds and 2 lone pairs
B) 5 bonds and 1 lone pair.
C) 4 bonds and 1 lone pair.
D) 5 bonds and 2 lone pairs.
E) none of these.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the three molecules below, which choice is correct? I. TeCl2 II. GeF4 III. SH2
A) In both I and II the central atom has more than eight electrons about it (an expanded octet) , III has 8 electrons around the S atom.
B) In I the Te atom has more than 8 electrons (an expanded octet) around it, II and III both place 8 electrons around the central atom.
C) In II the Ge atom has more than 8 electrons (an expanded octet) around it, I and III both place 8 electrons around the central atom.
D) In I, II, and III the central atom has more than 8 electrons (an expanded octet) around it.
E) In I, II, and III the central atom has 8 electrons around it.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the polyatomic ion, (SO3) 2 - . Which Lewis structure below obeys the octet rule for all atoms in the structure and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the Electron Dot formula for the oxide ion, O2 - ?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four compounds listed below represent an exception to the octet rule when determining their respective Lewis formulas. Which one of the following molecules follows the octet rule when determining its Lewis structure?
A) PF5
B) BeCl2
C) NO
D) CO2
E) XeF4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A possible Lewis structure for BeCl₂ is given below. 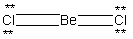 Why does this resonance form contribute very little to the true electronic structure for this molecule?
Why does this resonance form contribute very little to the true electronic structure for this molecule?
A) It does not use the proper number of valence electrons.
B) It does not obey the octet rule for Beryllium.
C) It does not obey the octet rule for Chlorine.
D) The formal charge of the Beryllium atom is +2 and the formal charge of each Chlorine atom is - 1. This provides a charge separated compound.
E) The formal charge of the Beryllium atom is - 2 and the formal charge of each Chlorine atom is +1. This places a negative charge on a metal and a positive charge on a highly electronegative non-metal atom.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis structure below obeys the octet rule for every atom in the structure of N2 and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A possible Lewis structure for BF 3 is given below. 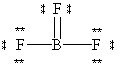 Why does this resonance form contribute very little to the true electronic structure for this molecule?
Why does this resonance form contribute very little to the true electronic structure for this molecule?
A) The formal charge of the Boron atom is +3 and the formal charge of each Flourine atom is - 1. This provides a charge separated compound.
B) The formal charge of the Boron atom is - 1 and the formal charge of the top Flourine atom is +1. This places a negative charge on a metal and a positive charge on a highly electronegative non-metal atom.
C) It does not obey the octet rule for Boron.
D) It does not obey the octet rule for each Flourine.
E) It does not use the proper number of valence electrons.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds would be classified as polar covalent ?
A) H - H
B) H - Cl
C) Cl - Cl
D) Na - Cl
E) all of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the expected bond order for each C - C bond in C6H6 (the carbon atoms are arranged in a ring with one hydrogen atom bonded to each) ?
A) 1
B) 2
C) 3
D) 1.5
E) none of these
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Below are two resonance forms of nitrogen monoxide. Which of the two structures is the preferred resonance form and why?
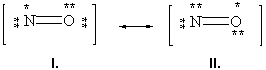
A) Structure I is the preferred structure because the formal charge of each atom in the structure equals zero.
B) Structure I is the preferred structure because it obeys the octet rule for each atom in the structure.
C) Structure II is the preferred structure because the formal charge of each atom in the structure equals zero.
D) Structure II is the preferred structure because it obeys the octet rule for each atom in the structure.
E) These are equivalent resonance forms.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species listed below has the largest lattice energy?
A) NaCl
B) LiCl
C) LiF
D) KBr
E) KF
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis structure below obeys the octet rule for every atom in the structure of NBr3 and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three possible resonance forms for NO₂ NH - are shown below. Pick the best answer.
I. 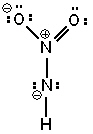 II.
II.  III.
III. 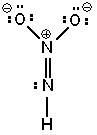
A) I and II are correct resonance forms, III is not and no others exist
B) I and II are not correct resonance forms, III is the only possible Lewis structure for this molecule
C) None of the Lewis structures shown are correct; one or more other Lewis structure(s) can be written.
D) All of the Lewis structures are correct resonance forms and no others exist.
E) All of the Lewis structures are correct resonance forms but one or more others can also be written.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 119
Related Exams