Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following units is the relative mass of a molecule expressed in atomic mass units and is calculated by adding together the atomic weights of the atoms in the molecule?
A) Atomic number
B) mass number
C) Mass number
D) Atomic weight
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms that have the same atomic number but differ by mass number are called?
A) protons
B) neutrons
C) isotopes
D) positrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following units can be used to collect a sample of atoms of one element with a mass in grams equal to the atomic weight of another element?
A) Molecular weight
B) Avogadro's number
C) Atomic number
D) Atomic mass
Correct Answer

verified
Correct Answer
verified
True/False
Calcium supplements can be taken in 1,000 mg increments.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are found around the species below? 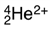
A) -2
B) 0
C) 2
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is wrong with the following molecular formula: SOO (sulfur dioxide) ?
A) OSO is the correct form
B) SO should be So
C) OO should be written as O2
D) OO should be written as O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
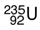 is the form of uranium used to make atomic bombs. One atom of this isotope consists of ___.
is the form of uranium used to make atomic bombs. One atom of this isotope consists of ___.
A) 92 protons, 92 electrons, 92 neutrons
B) 92 protons, 143 electrons, 92 neutrons
C) 92 protons, 92 electrons. 143 neutrons
D) 143 protons, 143 electrons, 92 neutrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to a periodic table and tell how many helium atoms (He) would be needed to get close to the same mass as an average oxygen atom (O) .
A) six
B) four
C) twelve
D) one-fourth
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If calcium carbonate found in limestone is 40.0% calcium, how many grams of calcium are in 485 g of calcium carbonate?
A) 12.1 g of calcium
B) 291 g of calcium
C) 19,400 g of calcium
D) 194 g of calcium
Correct Answer

verified
Correct Answer
verified
True/False
The symbols for all of the elements are derived from the Latin names.
Correct Answer

verified
Correct Answer
verified
True/False
One mole of any substance will contain one Avogadro's number of atoms of that substance.
Correct Answer

verified
Correct Answer
verified
True/False
One mole of O3 weighs 16 grams.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass number of a carbon-13 (13C) atom?
A) 13
B) 12
C) 6
D) 7
Correct Answer

verified
Correct Answer
verified
True/False
One mole of an element would weigh the same as a mole of an isotope of the same element.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many neutrons are in an atom that has a mass number of 75 and contains 35 protons?
A) 40
B) 35
C) 75
D) can't tell
Correct Answer

verified
Correct Answer
verified
True/False
A mole of copper contains the same number of atoms as a mole of zinc.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly describes subatomic particles? 1. Mass: 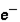 <
< 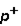 = n
2. Magnitude of charge: n
= n
2. Magnitude of charge: n 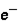 =
= 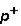 3. Location: outside nucleus
3. Location: outside nucleus 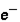 ,
, 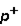 , inside nucleus n
, inside nucleus n
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
Correct Answer

verified
Correct Answer
verified
True/False
One mole of an element would weigh the same as a mole of an isotope of the same element.
Correct Answer

verified
Correct Answer
verified
True/False
A scanning tunneling microscope (STM)relies on a very strong light source to help see atoms.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 96
Related Exams