A) Coenzymes block enzyme activity.
B) Products of metabolic reactions block enzyme activity.
C) NADH is altered in electron transport chains.
D) ADP is phosphorylated.
E) Low reactant concentrations decrease enzyme activity.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the ADH reaction and answer the following question. ADH stands for alcohol dehydrogenase. 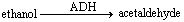 In this reaction, acetaldehyde represents the ____.
In this reaction, acetaldehyde represents the ____.
A) enzyme
B) reactant
C) product
D) activation energy
E) trigger
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Movement of substances that requires the expenditure of ATP molecules is called ____.
A) facilitated diffusion
B) simple diffusion
C) osmosis
D) active transport
E) bulk flow
Correct Answer

verified
Correct Answer
verified
Matching
Match the following questions about cell membrane permeability correctly.
Correct Answer
Multiple Choice
The process by which a cell takes in a small amount of extracellular fluid and its contents by the ballooning inward of the plasma membrane is the definition of ____.
A) endocytosis
B) exocytosis
C) phagocytosis
D) passive transport
E) osmosis
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The second law of thermodynamics states that ____.
A) the energy of the universe is a constant
B) energy can be neither created nor destroyed
C) energy disperses spontaneously
D) energy transformations create a more orderly universe
E) energy and matter are the same thing
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
If the activation energy for a chemical reaction is very high, the ____.
A) reaction will have an overall net gain of energy
B) reaction will have an overall net loss of energy
C) reaction will progress slowly
D) reaction will progress quickly
E) reactants have a lower energy than the products
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The amount of turgor that is enough to stop osmosis is called ____.
A) the wilting point
B) osmotic pressure
C) hypotonicity
D) expansion pressure
E) hypertonicity
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Osmosis is an example of ____.
A) facilitated diffusion
B) passive transport
C) active transport
D) exocytosis
E) endocytosis
Correct Answer

verified
Correct Answer
verified
Multiple Choice
F. acidarmanus , a species of bacteria that lives in California copper mines, has adapted to its environment by altering the ____ at which its enzymes function.
A) temperature
B) pH
C) light levels
D) substrate concentration
E) salt concentration
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cells store energy in the form of ____.
A) sunlight
B) chloroplasts
C) carbon dioxide
D) glucose
E) water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does wood keep burning once it is lit?
A) Intermediates from the reaction drive the reaction forward.
B) Energy is given off during the reaction that drives the reaction forward.
C) The products have more energy than the reactants, therefore driving the reaction forward.
D) The reactants have more energy than the products, therefore driving the reaction forward.
E) The activation of the energy drives the reaction forward.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Heating up a reaction increases the speed of a reaction until the ____.
A) enzyme denatures
B) heat changes the pH of the reaction
C) maximum speed is achieved
D) cofactors can ' t bind the enzyme
E) reaction begins to experience feedback inhibition
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A concentration gradient ceases to exist when ____.
A) all molecules have moved from low concentration to high concentration
B) the membrane pores close
C) the temperature drops
D) there is equilibrium between the two sides of a membrane
E) bulk flow intervenes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Regulatory factors that bind to enzymes ____.
A) always increase enzyme activity
B) always decrease enzyme activity
C) alter the shape of the enzyme
D) alter the pH at which the enzyme works
E) alter the temperature at which the enzyme works
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
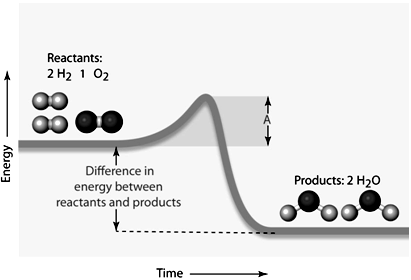 In the given figure, the amount of energy symbolized by the line "A" would be the ____.
In the given figure, the amount of energy symbolized by the line "A" would be the ____.
A) reactant energy
B) product energy
C) cofactor energy
D) activation energy
E) intermediate energy
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A series of enzyme-mediated reactions by which cells build, remodel, or break down organic molecules is known as ____.
A) energy carriers
B) metabolic pathways
C) the induced-fit model
D) intermediary compounds
E) activation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When ethanol (C2H5OH) and oxygen (O2) react together, they form carbon dioxide (CO2) and water (H2O) . The resulting chemical reaction is: C 2 H 5 OH + 3O 2 → 2CO 2 + 3H 2 O The coefficient in front of H2O indicates there are ____.
A) three oxygen atoms in the reaction
B) three carbon atoms in the water
C) three water molecules in the reaction
D) six water molecules in the reaction
E) six oxygen atoms in the reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The minimum amount of energy needed to get a chemical reaction started is called the ____ energy.
A) activation
B) reaction
C) enzymatic
D) chemical
E) triggering
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The initial event that triggers phagocytosis is when the ____.
A) solute concentration is higher inside the cell than outside
B) solute concentrations is lower inside the cell than outside
C) target protein binds to the receptor
D) enzymes bind to the membrane
E) vesicles bud off from the membrane
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 80
Related Exams