A) A
B) B
C) C
D) A and B
E) B and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 570. mm Hg and 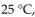 a gas sample has a volume of 2270 mL. What is the final pressure (in mmHg) at a volume of 1250 mL and a temperature of
a gas sample has a volume of 2270 mL. What is the final pressure (in mmHg) at a volume of 1250 mL and a temperature of 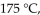 if the amount of gas does not change?
if the amount of gas does not change?
A) 690. mmHg
B) 1560 mmHg
C) 700 . mmHg
D) 470 . mmHg
E) 210. mmHg
Correct Answer

verified
Correct Answer
verified
Essay
Oxygen makes up about ________ percent of the atmosphere.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the kinetic molecular theory of gas behavior, the distance between gas molecules is assumed to be ________ the diameter of the gas molecules.
A) approximately the same as
B) 22.4 times
C) large relative to
D) dependent on
E) small relative to
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the combined gas law is rearranged to solve for 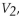 the following is the correct expression:
the following is the correct expression: 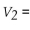
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
At STP, the molar volume of a gas is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the kinetic theory of gases, a gas can be compressed much more than a liquid or solid because
A) gas particles do not attract or repel one another.
B) a gas is composed of very small particles.
C) gas particles move faster when the temperature increases.
D) the particles of a gas are very far apart.
E) gas particles move rapidly.
Correct Answer

verified
Correct Answer
verified
Essay
One atmosphere is the same as ________ mmHg.
Correct Answer

verified
Correct Answer
verified
Essay
The ideal gas law is usually written as ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As the temperature of a gas increases, the density of the gas will
A) increase.
B) decrease.
C) remain constant.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An autoclave is used to sterilize surgical equipment because
A) it allows water to boil at temperatures above ![]()
B) it allows water to boil at ![]() at pressures greater than 1 atm.
at pressures greater than 1 atm.
C) it provides very high temperatures and very low pressures.
D) it allows water to boil at ![]() at pressures less than 1 atm.
at pressures less than 1 atm.
E) it allows water to boil at temperatures less than ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a potential use for a hyperbaric chamber?
A) counteracting carbon monoxide poisoning
B) treating a diver with the bends
C) treating some cancers
D) treatment for burns and infections
E) increasing the rate at which a broken bone heals
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm?
A) 1.00 atm
B) 633 atm
C) 127 atm
D) 1.20 atm
E) 0.833 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If atmospheric pressure on a certain day is 749 mmHg, what is the partial pressure of nitrogen, given that nitrogen is about 78% of the atmosphere?
A) 760 mmHg
B) 170 mmHg
C) 600 mmHg
D) 750 mmHg
E) 580 mmHg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of helium gas at 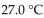 and 3.60 atm pressure is cooled in the same container to a temperature of
and 3.60 atm pressure is cooled in the same container to a temperature of 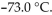 What is the new pressure, if volume and amount of gas do not change?
What is the new pressure, if volume and amount of gas do not change?
A) 5.40 atm
B) 2.40 atm
C) 2.38 atm
D) 3.12 atm
E) 4.15 atm
Correct Answer

verified
Correct Answer
verified
Essay
Avogadro's Law is usually written as ________.
Correct Answer

verified
Correct Answer
verified
Essay
Charles's law is usually written as ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Boyle's Law, the pressure of a gas increases as the volume decreases because
A) the gas particles strike the walls of the container with more force.
B) the kinetic energy of the gas particles increases.
C) the temperature of the gas increases.
D) the gas particles strike the walls of the container more often.
E) the gas particles get bigger.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A diver exhales a bubble with a volume of 250 mL at a pressure of 2.4 atm and a temperature of 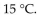 What is the volume of the bubble when it reaches the surface where the pressure is 1.0 atm and the temperature is
What is the volume of the bubble when it reaches the surface where the pressure is 1.0 atm and the temperature is 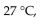 if the amount of gas does not change?
if the amount of gas does not change?
A) 110 mL
B) 100 mL
C) 1100 mL
D) 580 mL
E) 630 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A tank contains a mixture of helium, neon, and argon gases. If the total pressure in the tank is 490. mmHg and the partial pressures of helium and argon are 215 mmHg and 102 mmHg, respectively, what is the Partial pressure of neon?
A) 377 mmHg
B) 173 mmHg
C) 603 mmHg
D) 0.228 mmHg
E) 807 mmHg
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 84
Related Exams