A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) seesaw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules is nonpolar?
A) NH3
B) OF2
C) CH3Cl
D) H2O
E) BeCl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule with the formula AB4 and a square planar molecular geometry uses ________ to form its σ bonds.
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals
Correct Answer

verified
Correct Answer
verified
Essay
In one sentence state how molecular orbitals are usually obtained.
Correct Answer

verified
Molecular orbitals are obtaine...View Answer
Show Answer
Correct Answer
verified
View Answer
True/False
In the valence bond treatment, overlap of an s orbital on one atom with an sp3 orbital on another atom gives rise to a σ bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predicted molecular geometry of the CH4 molecule according to the VSEPR model?
A) tetrahedral
B) trigonal pyramidal
C) trigonal planar
D) square planar
E) seesaw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model, predict the molecular geometry around the central atom in SO32-.
A) trigonal planar
B) trigonal pyramidal
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bond angles in the H3O+ ion are
A) 120°.
B) 90°, 120°.
C) 109°.
D) 90°.
E) 180°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of ClO3F as predicted by the VSEPR model? 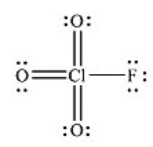
A) trigonal pyramidal
B) square planar
C) square pyramidal
D) tetrahedral
E) octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of NOCl as predicted by the VSEPR model? 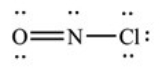
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predicted molecular geometry of the H2O molecule according to the VSEPR model?
A) tetrahedral
B) trigonal pyramidal
C) bent
D) square planar
E) seesaw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the total number of electron domains around the central atom for a molecule having a square planar molecular geometry, such as XeBr4?
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most reasonable prediction for the H-C-H bond angle in CH4?
A) 90°
B) 109.5°
C) 120°
D) 107°
E) 105°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR model, which molecule is predicted to be linear?
A) H2S
B) HCN
C) BF3
D) H2CO
E) SO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization on the central atom in NO3-?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
True/False
When molecules that exhibit separation of charges are placed in an electric field, they become oriented positive end to negative plate and negative end to positive plate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule with the formula AB4 and a tetrahedral molecular geometry uses ________ to form its σ bonds.
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For different structural arrangements of atoms having the formula XeF2Cl2, which structures represent polar molecules? (Black = Xe, Yellow = F, Green = Cl) 
A) I and III
B) II only
C) I, II and III
D) II and III
E) None of these structures are polar.
Correct Answer

verified
Correct Answer
verified
True/False
Pi bonds are covalent bonds in which the electron density is concentrated above and below the plane of the nuclei of the bonding atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR model, the predicted molecular geometry of SiCl4 is
A) linear.
B) trigonal planar.
C) bent.
D) tetrahedral.
E) trigonal pyramidal.
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 139
Related Exams