A) H2SO3, SCl5, C2H5
B) H2SO4, SCl5, C2H5
C) H2SO4, SCl6, C2H6
D) H2SO4, SCl6, CH6
E) H2SO3, SCl6, C2H6
Correct Answer

verified
Correct Answer
verified
True/False
Molarity is defined as the moles of solute divided by the liters of solvent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding empirical formulas, molecular formulas, and percent composition is correct?
A) The compounds NO2 and N2O4 have the same empirical formula.
B) The compounds NO2 and N2O4 have the same molecular formula.
C) The empirical formula of H2O2 is H2O.
D) The empirical formula of N2O5 is NO2.5.
E) The compounds PCl3 and PCl5 would have the same percent composition.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many formula units are there in 2.5 moles of MgCl2?
A) 2.5
B) 7.5
C) 1.5 × 1024
D) 4.5 × 1024
E) 4.2 × 10−24
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the moles and the mass of solute in 250.0 mL of 6.00 M NaOH.
A) 6.00 moles and 240.
B) 1.50 moles and 60.0 g
C) 1.50 moles and 0.0375 g
D) 6.00 moles and 0.0150 g
E) 24.0 moles and 0.600 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of 3.5 × 1023 molecules of CO2?
A) 1.7 g
B) 26 g
C) 0.58 g
D) 75 g
E) 1.5 × 1025 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order from least chlorine atoms to most chlorine atoms in a 50.0 g sample: Cl2, ClF3, Cl2O, PCl3
A) ClF3 < Cl2O < Cl2 < PCl3
B) PCl3 < Cl2O < Cl2 < ClF3
C) ClF3 < PCl3 < Cl2O < Cl2
D) ClF3 < PCl3 < Cl2 < Cl2O
E) Cl2O < Cl2 < ClF3 < PCl3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the molar mass of a substance is 20.0 g/mol, what is the mass of 3.01 × 1025 molecules of the substance?
A) 0.400 g
B) 10.0 g
C) 1.00 × 103 g
D) 6.02 × 1026 g
E) 1.20 × 1025 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following molecular formulas, determine the empirical formula of each compound: P4O10, SnCl2, N2O3, CH3CO2H.
A) P2O5, SnCl2, N2O3, CH3CO2H
B) PO2.5, SnCl2, N2O3, CH3CO2H
C) P2O5, SnCl2, N2O3, CH2O
D) PO2.5, SnCl, NO1.5, CH3CO2H
E) P4O10, SnCl4, N2O3, CH2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molarity of a solution consisting of 65.5 g of K2SO4 in 5.00 L of solution.
A) 3.81 × 103 M
B) 0.250 M
C) 0.0752 M
D) 0.376 M
E) 0.125 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding empirical and molecular formulas is correct?
A) Information on percent composition is all that is necessary to determine both an empirical and a molecular formula.
B) A compound with an empirical formula of CH2O and a molar mass of 90 g/mol would have a molecular formula of C3H6O3.
C) The compounds C2H4 and C3H6 have different empirical formulas.
D) The formula Ca2Cl4 is the correct empirical formula for calcium chloride.
E) The compound benzene has a molecular formula of C6H6, so its empirical formula would be C3H3.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the substances in the figure from least atoms per mole to most atoms per mole. 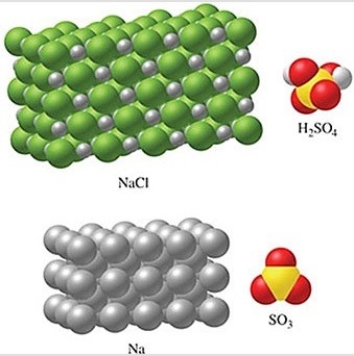
A) SO3 < H2SO4 < NaCl < Na
B) Na < NaCl < SO3 < H2SO4
C) Na < NaCl < H2SO4 < SO3
D) NaCl < Na < SO3 < H2SO4
E) H2SO4 < SO3 < NaCl < Na
Correct Answer

verified
Correct Answer
verified
True/False
To obtain 0.50 moles of potassium nitrate from a solution, you could either use 100.0 mL of a 5.0 M solution, or 250.0 mL of a 2.0 M solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 5.05 g sample of quartz (SiO2) contains 2.36 g of silicon. What are the percentages of silicon and oxygen in quartz?
A) 53.3% Si and 46.7% O
B) 46.7% Si and 53.3% O
C) 29.9% Si and 70.1% O
D) 70.1% Si and 29.9% O
E) 46.7% Si, and insufficient information to calculate % O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass of HCl.
A) 36.46 g/mol
B) 13.02 g/mol
C) 2.196 × 1025 g/mol
D) 6.054 × 10-23 g/mol
E) 72.92 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass of Na2SO4.
A) 94.05 g/mol
B) 71.06 g/mol
C) 119.06 g/mol
D) 142.04 g/mol
E) 110.05 g/mol
Correct Answer

verified
Correct Answer
verified
Short Answer
Calculate the number of moles provided by 50.0 mL of 3.00 M NaOH solution.
Correct Answer

Answered by ExamLex AI
To calculate the number of moles of NaOH...View Answer
Show Answer
Correct Answer
Answered by ExamLex AI
View Answer
Multiple Choice
How many oxygen atoms are there in 0.25 mole of CO2?
A) 0.50
B) 0.25
C) 3.0 × 1023
D) 1.5 × 1023
E) 4.2 × 10−25
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following in order of increasing mass: 1.0 mole of methane (CH4) , 0.50 mole of water (H2O) , 0.20 mole of Fe, and 0.010 mole of U.
A) U < Fe < H2O < CH4
B) H2O < CH4 < Fe < U
C) H2O < CH4 < U < Fe
D) U < H2O < CH4 < Fe
E) U < H2O < Fe < CH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 6.25 g sample of magnetite (Fe3O4) contains 4.52 g of Fe. What are the percentages of iron and oxygen in magnetite?
A) 28.6% Fe and 71.4% O
B) 42.8% Fe and 57.2% O
C) 72.3% Fe and 27.7% O
D) 27.7% Fe and 72.3% O
E) 57.2% Fe and 42.8% O
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 144
Related Exams