A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assuming that no other particles are produced, which of the following particles could be used to bombard nitrogen-14 in order to make fluorine-18?
A) Alpha particle
B) Beta particle
C) Neutron
D) Proton
E) Positron
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain isotope has a specific activity of 7.29 × 10-4 Ci/g. How many α particles will a 75.0 mg sample emit in one hour?
A) 9.99 × 10 4
B) 2.02 × 10 6
C) 7.28 × 10 9
D) 1.29 × 10 12
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 55-kg person exposed to thorium-234 receives 7.5 × 104 β particles, each with an energy of 1.6 × 10-14 J. How many rads does the person receive?
A) 2.1 × 10 -19
B) 1.2 × 10 -17
C) 2.2 × 10 -9
D) 1.2 × 10 -9
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
It is believed that two carbon-12 nuclei can react in the core of a supergiant star to form sodium-23 and hydrogen-1. Calculate the energy released from this reaction for each mole of hydrogen formed. The masses of carbon-12, sodium-23, and hydrogen-1 are 12.0000 amu, 22.989767 amu, and 1.007825, respectively. 
A) 2.16 × 10 14 kJ
B) 2.16 × 10 11 kJ
C) 2.16 × 10 8 kJ
D) 2.16 × 10 5 kJ
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following equations correctly represents electron capture by the  nucleus?
nucleus?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An isotope with Z > 83, which lies close to the band of stability, will generally decay through
A) α decay.
B) β decay.
C) γ decay.
D) positron decay.
E) electron capture.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following elements is formed largely in supernova explosions?
A) H
B) He
C) Mg
D) Fe
E) U
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclide Pb-210 undergoes three successive decays (beta, alpha, and beta, respectively) to form a stable nuclide. What are the three nuclides that form from Pb-210 in this decay series?
A) Tl-210, Au-206, Pt-206
B) Bi-210, Tl-206, Pb-206
C) Pb-209, Hg-205, Hg-204
D) Bi-210, Pb-206, Bi-206
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 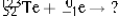
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cesium-134 is a β emitter with a half-life of 2.0 years. How much of a 2.50-g sample of cesium-134 will remain after 10 years?
A) 0.0024 g
B) 0.078 g
C) 0.25 g
D) 0.50 g
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 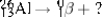
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is an incorrect representation of the indicated particle or nucleus?
A) Positron: ![]()
B) Neutron: ![]()
C) Helium-3: ![]()
D) Alpha particle: ![]()
E) Proton: ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the specific activity (in Ci/g) of an isotope if 3.56 mg emits 4.26 × 108 β particles per second?
A) 0.003232 Ci/g
B) 0.0115 Ci/g
C) 0.309 Ci/g
D) 3.23 Ci/g
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Palladium-107 undergoes β decay (t1/2 = 6.5 × 105 yr) to form silver-107. How long will it take for 0.150 mol of silver-107 to form from 1.25 mol of palladium-107?
A) 2.0 × 10 7 y
B) 1.4 × 10 7 y
C) 1.2 × 10 6 y
D) 8.3 × 10 5 y
E) 1.2 × 10 5 y
Correct Answer

verified
Correct Answer
verified
True/False
Positron decay and electron capture have the same net effect on the Z and N values of a nucleus.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following descriptions relating to nuclear reactions is correct?
A) The ratio of neutrons to protons remains constant.
B) The number of protons plus neutrons remains constant.
C) The number of electron remains constant.
D) The total charge changes.
E) The total number of nucleons changes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 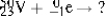
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation. 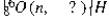
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 82
Related Exams