A) Cs
B) SO2
C) I2
D) CCl4
E) Ni
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the pair of substances which has the one with the higher equilibrium vapor pressure at a given temperature listed first.
A) H2O, H2S
B) CH3CH2OH, CH3OH
C) CH3COCH3, CH3CH2CH2OH
D) NH3, PH3
E) Br2, Cl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the type of solid shown in the image. 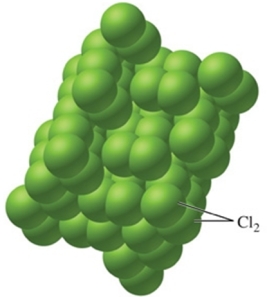
A) metallic
B) ionic
C) network
D) nonpolar molecular
E) polar molecular
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not an attractive force that acts between the individual molecules of CH3OH?
A) London dispersion forces
B) dipole-dipole forces
C) hydrogen-bonding forces
D) covalent bonds
E) none of these is correct
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pictured change of state occurs at constant temperature even though heat is being added.Where does this change of state occur on the cooling curve? 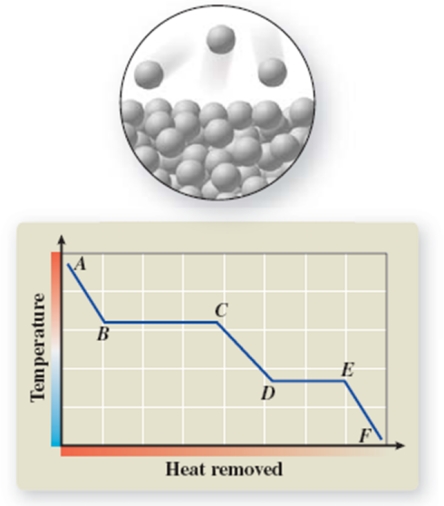
A) AB
B) BC
C) CD
D) DE
E) EF
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following substances in order of increasing intermolecular forces: Ar, H2O, N2, O2
A) Ar < H2O < N2 < O2
B) Ar < H2O < O2 < N2
C) H2O < N2 < O2 < Ar
D) N2 < O2 < Ar < H2O
E) Ar < N2 < O2 < H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The physical properties of a substance influenced by the strength of intermolecular forces include all of the following except:
A) melting point.
B) boiling point.
C) viscosity.
D) vapor pressure.
E) mass.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which substance has the highest melting point?
A) Cu
B) C6H14
C) Rn
D) C (graphite)
E) I2
Correct Answer

verified
Correct Answer
verified
True/False
Network covalent substances have the highest melting points of all substances.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding intermolecular forces is incorrect?
A) An intermolecular force is an attractive force that operates between molecules.
B) Bonding forces are much stronger than intermolecular forces.
C) London dispersion forces occur in all atoms and molecules.
D) London dispersion forces are the result of permanent dipoles in atoms or molecules.
E) Molecules which have hydrogen bonded to F, O, or N can undergo hydrogen-bonding.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atmospheric pressure on Mount Everest is about 0.30 atm.From the vapor pressure curve of propane, it can be seen the boiling point of propane at this elevation is about _________. 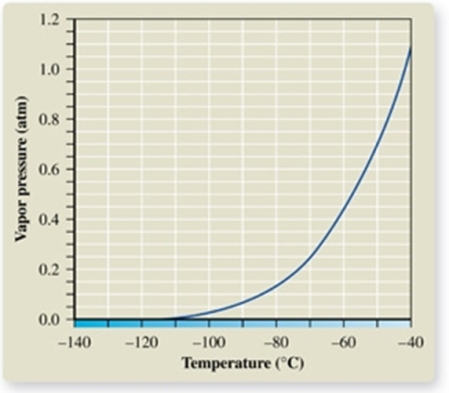
A) -137oC
B) -120oC
C) -67oC
D) -42oC
E) 0oC
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is correct?
A) The phase change from solid to liquid is exothermic.
B) Liquids boil at lower than normal temperatures in Death Valley, since it is below sea level.
C) A substance with a low boiling point would be expected to have a relatively low vapor pressure at a low temperature.
D) The phase change from liquid to gas is exothermic.
E) When a substance condenses, heat energy is given off.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the vapor pressure curve of acetone, it can be seen that the normal boiling point of acetone is about _________. 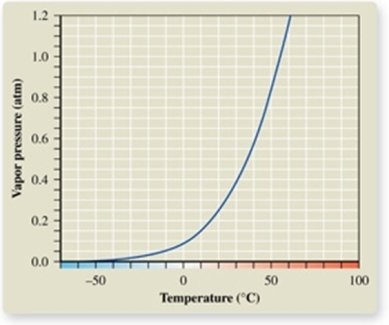
A) -50oC
B) 0oC
C) 50oC
D) 57oC
E) 100oC
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the type of solid shown in the image. 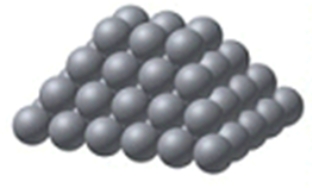
A) metallic
B) ionic
C) network
D) nonpolar molecular
E) polar molecular
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules experience dipole-dipole forces?
A) CO2
B) SO2
C) CCl4
D) H2S
E) both SO2 and H2S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the melting point of the following solids.Which of these solids is most likely a network solid?
A) SiF4, -90oC
B) CO2, -57oC
C) BN, 3000oC
D) KBr, 734oC
E) GeBr4, 26oC
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the amount of heat energy required to convert 35.0 g of ice at -12.5 C to water at 24.0 C.(Cwater = 4.18 J/g C; Cice = 2.03 J/g C; molar heat of fusion of ice = 6.01 x 103 J/mol)
A) 8.88 x 102 J
B) 1.17 x 104 J
C) 1.61 x 1010 J
D) 3.51 x 103 J
E) 1.61 x 104 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding the liquid state is incorrect?
A) Liquids are about 1000 times denser than gases.
B) Liquids are normally about 90-95% as dense as their corresponding solid.
C) The viscosity of a liquid is a measure of its resistance to flow.
D) Molecules with high intermolecular forces will have high surface tension.
E) Surface tension increases as temperature increases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What phase transition is occurring between points B and C on the heating curve? 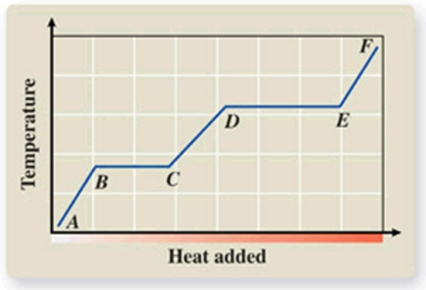
A) melting
B) condensation
C) evaporation
D) sublimation
E) deposition
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the highest boiling point?
A) H2
B) HF
C) HCl
D) HBr
E) HI
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 107
Related Exams