A) Loss of a proton from a base forms its conjugate acid.
B) Loss of a proton from an acid forms its conjugate base.
C) Gain of a proton by an acid forms its conjugate base.
D) Brønsted-Lowry acid-base reactions always result in the transfer of a proton from a base to an acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of increasing acidity, putting the least acidic first. 
A) I < IV < III < II
B) I < III < IV < II
C) II < III < IV < I
D) II < IV < III < I
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following concepts can be used to explain the difference in acidity between acetic acid (CH3COOH) and ethanol (CH3CH2OH) ?
A) Hybridization
B) Electronegativity
C) Resonance
D) Size
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following ranks the compounds in order of increasing basicity, putting the least basic first?
A) CH3NH2 < CH3OH < CH4
B) CH3OH < CH3NH2 < CH4
C) CH4 < CH3NH2 < CH3OH
D) CH4 < CH3OH < CH3NH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the direction of equilibrium when acetylene (C2H2) reacts with H2N- in an acid-base reaction?

A) Left
B) Right
C) Neither
D) Cannot be determined
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the direction of equilibrium when acetylene (C2H2) reacts with ethoxide (CH3CH2O-) in an acid-base reaction?

A) Left
B) Right
C) Neither
D) Cannot be determined
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What are the products of the following proton transfer reaction?

A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species cannot act as both a Brønsted-Lowry acid and base?
A) HCO3-
B) HSO4-
C) HO-
D) H2PO4-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of decreasing acidity, putting the most acidic first. 
A) IV > II > III > I
B) III > II > IV > I
C) I > II > IV > III
D) III > IV > II > I
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species is not a Brønsted-Lowry base?
A) BF3
B) NH3
C) H2O
D) PO43-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is both a Brønsted-Lowry acid and base?

A) I, II
B) I, III
C) II, IV
D) I, IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about Lewis bases is true?
A) Lewis bases are electron pair acceptors.
B) Lewis bases are electron pair donors.
C) Lewis bases are proton donors.
D) Lewis bases are proton acceptors.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following concepts can be used to explain the difference in acidity between ethanol (CH3CH2OH) and 2-fluoroethanol (FCH2CH2OH) ?
A) Size
B) Inductive effect
C) Resonance
D) Hybridization
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species is the strongest base?
A) HO-
B) H2N-
C) CH3COO-
D) Cl-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the role of methylchloride (CH3Cl) in the following reaction?

A) Lewis acid
B) Lewis base
C) Brønsted-Lowry acid
D) Brønsted-Lowry base
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct rank of the following compounds in order of increasing acidity?
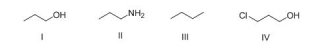
A) I > II > III > IV
B) IV > III > II > I
C) IV > I > II > III
D) III > I > IV > II
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Rank the following conjugate bases in order of increasing basicity, putting the least basic first. 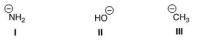
A) II < I < III
B) II < III < I
C) I < II < III
D) I < III < II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid?
A) CH3OH
B) BrCH2OH
C) CH3NH2
D) CH3Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following molecule with protons labeled, I-III.Rank these protons in order of decreasing acidity, putting the most acidic first. 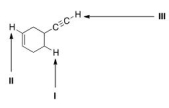
A) I > II > III
B) I > III > II
C) III > II > I
D) III > I > II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is not a Lewis acid?
A) AlCl3
B) HCl
C) H2O
D) CBr4
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 52
Related Exams