Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many carbon-carbon sigma bonds are in the following compound? 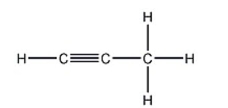
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many sp2 carbons are present in H2C=C=CH2
A) 0
B) 1
C) 1.5
D) 2
E) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes the third shell that surrounds the nucleus of an atom?
A) The third shell contains only s and p atomic orbitals.
B) The maximum number of electrons that can occupy the third shell is 18.
C) The total number of atomic orbitals present in the third shell is 16.
D) The third shell can contain f orbitals.
E) All third shell elements must have d electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the electronic configuration of the element Fe?
A)
B)
C)
D)
E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Are all the carbons in this structure in the same plane? 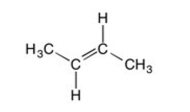
A) Yes
B) No
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge on the nitrogen in the ammonium ion?
A) -2
B) -1
C) 0
D) +1
E) +2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the smallest dipole moment?
A) Br2
B) NH3
C) HCl
D) HBr
E) HI
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the carbon H2CO
A) sp
B) sp2
C) sp3
D) sp4
E) s3p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following covalent bonds has the largest dipole moment?
A) C-C
B) C-H
C) C-O
D) H-N
E) H-F
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formal charge on nitrogen in the following compound is ________.
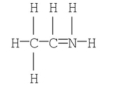
A) +2
B) +1
C) 0
D) -1
E) -2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the bond angle in the following compound?
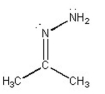
A) ~60°
B) ~90°
C) ~110°
D) ~120°
E) ~ 180°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the hydrogen halides, the strongest bond is found in ________ and the longest bond is found in ________.
A) HF; HF
B) HF; HI
C) HI; HF
D) HI; HI
E) HCl; HBr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The carbon-carbon double bond in ethene is ________ and ________ than the carbon-carbon triple bond in ethyne.
A) stronger; shorter
B) stronger; longer
C) weaker; shorter
D) weaker; longer
E) stronger; more polar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following contains polar covalent bonds?
A) NH3
B) Na2O
C) H2
D) KF
E) both A and C
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the hybridization of the nitrogen in (CH3)3N
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the weakest bond?
A) H2
B) HF
C) HCl
D) HBr
E) HI
Correct Answer

verified
Correct Answer
verified
Essay
What two factors determine the size of a bond's dipole moment?
Correct Answer

verified
The size of the char...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What orbitals overlap to form the H-C bond in CH3+?
A) sp3-sp3
B) sp2-sp3
C) s-p
D) s-sp2
E) s-sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound CH3NH2, contains a C-N bond. Which of the following best describes the charge on the nitrogen atom in this compound?
A)
B) slightly positive
C) uncharged
D) slightly negative
E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 74
Related Exams