A) bent
B) see-saw
C) trigonal planar
D) T-shaped
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Essay
For the chlorate ion, ClO3¯, draw two different valid Lewis structures, as follows: A) a structure in which the octet rule is obeyed. B) a structure in which formal charges are minimized.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of ClF2¯ as predicted by the VSEPR theory?
A) linear
B) bent
C) see-saw
D) T-shaped
E) L-shaped
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following Lewis structure for ClO3F, chlorine has a formal charge of ____ and an oxidation number of ____. 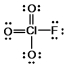
A) 7, 7
B) 7, -1
C) 1, 1
D) 1, -1
E) 1, 7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules and ions will have a planar geometry?
A) PCl3
B) BF4¯
C) XeF4
D) BrF5
E) H3O+
Correct Answer

verified
Correct Answer
verified
Essay
The Lewis structure of formaldehyde, CH2O, is shown. Use VSEPR theory to predict the molecular geometry and the H-C-H bond angle. Outline your reasoning. 
Correct Answer

verified
There are three electron groups around t...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Predict the ideal bond angles in IF2¯ using the molecular shape given by the VSEPR theory.
A) 60
B) 90
C) 109
D) 120
E) 180
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following structures does the central atom have a formal charge of +2?
A) SF6 ![]()
B) SO42¯ ![]()
C) O3 ![]()
D) BeCl2 ![]()
E) AlCl4¯ ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX2E2 will have a _____ molecular shape.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) see-saw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of BeH2 as predicted by the VSEPR theory?
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles around carbon in C2I2 using the molecular shape given by the VSEPR theory.
A) 90
B) 109
C) 120
D) 180
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best Lewis structure for ClCN.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX4E2 will have a _____ molecular shape.
A) tetrahedral
B) square pyramidal
C) square planar
D) octahedral
E) see-saw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct Lewis structure for NOCl, a reactive material used as an ionizing solvent.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX3E will have a _____ molecular shape.
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) triangular
Correct Answer

verified
Correct Answer
verified
Essay
List the three important ways in which molecules can violate the octet rule, and in each case draw one Lewis structure of your choice as an example.
Correct Answer

verified
Electron-deficient molecules have fewer ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Predict the ideal bond angles in AsCl3 using the molecular shape given by the VSEPR theory.
A) 90
B) 109
C) 120
D) 180
E) between 110 and 120
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of BCl3 as predicted by the VSEPR theory?
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following molecules are all the bonds single?
A) O3
B) POCl3
C) CO
D) COCl2
E) N2H4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles in GeCl4 using the molecular shape given by the VSEPR theory.
A) 90
B) 109
C) 120
D) 180
E) < 90
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 108
Related Exams