A) 1.75 * 105
B) 3.50
C) 0.286
D) 5.71 *10¯6
E) 1.40 * 10¯10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the mass-action expression, Qc, for the following chemical reaction. 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
SO2 reacts with O2 to produce SO3. If 86.0 g of SO2 is placed in a reaction vessel along with excess oxygen gas, how many moles of SO2 remain when 50.0 g of SO3 have been formed?
A) 0.56 mol
B) 0.62 mol
C) 0.72 mol
D) 0.78 mol
E) 1.34 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrogen sulfide can be formed in the following reaction: 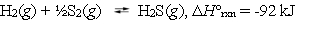 The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
A) 5.05
B) 18.8
C) 34.7
D) 88.9
E) 598
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant for reaction (1) below is 276. Under the same conditions, what is the equilibrium constant of reaction (2) ?
A) 6.02 * 10¯2
B) 7.25 * 10¯3
C) 3.62 *10¯3
D) 1.31 * 10¯5
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction system 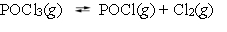 is at equilibrium. Which of the following statements describes the behavior of the system if POCl is added to the container?
is at equilibrium. Which of the following statements describes the behavior of the system if POCl is added to the container?
A) The forward reaction will proceed to establish equilibrium.
B) The reverse reaction will proceed to establish equilibrium.
C) The partial pressures of POCl3 and POCl will remain steady while the partial pressure of chlorine increases.
D) The partial pressure of chlorine remains steady while the partial pressures of POCl3 and POCl increase.
E) The partial pressure of chlorine will increase while the partial pressure of POCl decreases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
(p. Various sections) Which of the following has an effect on the magnitude of the equilibrium constant?
A) removing products as they are formed
B) adding more of a reactant
C) adding a catalyst
D) increasing the pressure, in a gas-phase reaction
E) change in temperature
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures. 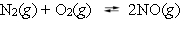 The equilibrium constant Kp for the reaction is 0.0025 at 2127 C. If a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of nitrogen?
The equilibrium constant Kp for the reaction is 0.0025 at 2127 C. If a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of nitrogen?
A) 0.16 atm
B) 0.31 atm
C) 3.1 atm
D) 7.7 atm
E) 7.8 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given this equilibrium constant data at 25 C, 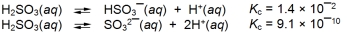 what is the value of Kc at this temperature for the reaction
what is the value of Kc at this temperature for the reaction 
A) 6.5 * 10¯8
B) 1.3 * 10¯11
C) 7.8 *1010
D) 1.5 * 107
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant for the reaction of bromine with chlorine to form bromine monochloride is 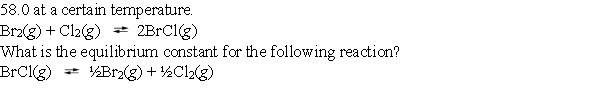
A) 2.97 *10¯4
B) 1.72 *10¯2
C) 3.45 * 10¯2
D) 1.31 *10¯1
E) > 1.00
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass-action expression, Qc, for the following chemical reaction? 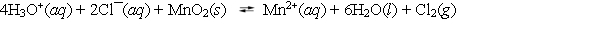
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
True/False
If all of the coefficients in the balanced equation for an equilibrium reaction are doubled, then the value of the equilibrium constant, Kc, will also be doubled.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 500 C, the equilibrium constant, Kp, is 4.00 *10¯4 for the equilibrium: 
A) 2.00* 10¯4
B) -4.00*10¯4
C) 1.25 *103
D) 2.50 * 103
E) 4.00 * 104
Correct Answer

verified
Correct Answer
verified
True/False
For some gas-phase reactions, Kp = Kc.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a chemical system is at equilibrium,
A) the concentrations of the reactants are equal to the concentrations of the products.
B) the concentrations of the reactants and products have reached constant values.
C) the forward and reverse reactions have stopped.
D) the reaction quotient, Q, has reached a maximum.
E) the reaction quotient, Q, has reached a minimum.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrogen sulfide will react with water as shown in the following reactions. 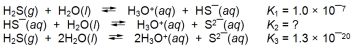 What is the value of K2?
What is the value of K2?
A) 1.3 * 10¯27
B) 2.3 *10¯7
C) 1.3 * 10¯13
D) 7.7 *1012
E) 7.7 * 1026
Correct Answer

verified
Correct Answer
verified
Multiple Choice
About half of the sodium carbonate produced is used in making glass products because it lowers the melting point of sand, the major component of glass. When sodium carbonate is added to water, it hydrolyses according to the following reactions. 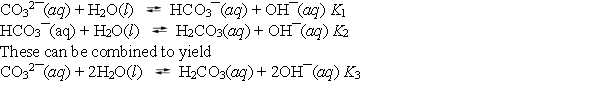 What is the value of K3?
What is the value of K3?
A) K1 *K2
B) K1 ¸ K2
C) K1 + K2
D) K1 - K2
E) (K1K2) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant, Kp, has a value of 6.5 *10¯4 at 308 K for the reaction of nitrogen monoxide with chlorine.  What is the value of Kc?
What is the value of Kc?
A) 2.5 * 10¯7
B) 6.5 * 10¯4
C) 1.6 * 10¯2
D) 1.7
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A container was charged with hydrogen, nitrogen, and ammonia gases at 120 C and the system was allowed to reach equilibrium. What will happen if the volume of the container is increased at constant temperature? 
A) There will be no effect.
B) More ammonia will be produced at the expense of hydrogen and nitrogen.
C) Hydrogen and nitrogen will be produced at the expense of ammonia.
D) The equilibrium constant will increase.
E) The equilibrium constant will decrease.
Correct Answer

verified
Correct Answer
verified
True/False
Unless H rxn = 0, a change in temperature will affect the value of the equilibrium constant Kc.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 102
Related Exams