A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom, X, has a tetrahedral electron geometry but a trigonal pyramidal molecular shape. How many atoms is atom X bonded to?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In this covalent molecule of methane, 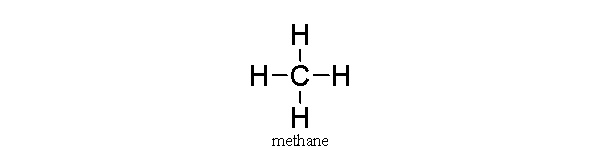
A) carbon is sharing its four electrons with the hydrogens.
B) carbon has donated four electrons to hydrogen, leaving it with a charge of +4.
C) hydrogen has eight electrons in its valence shell.
D) carbon is very unstable.
E) carbon does not have an octet of electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Below are two ions (not drawn to scale) . What will they do when in close proximity to one another? 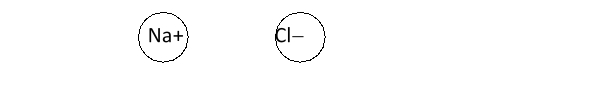
A) They will attract one another, forming an ionic compound.
B) They will attract one another, forming a covalent bond.
C) The sodium ion will transfer a proton to chloride.
D) The chloride ion will transfer an electron to sodium.
E) They will do nothing.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of the carbon indicated with arrow I in this structure of caffeine? 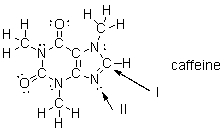
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) linear
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iron(II) is an example of a(n) ________.
A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In addition to single bonds, which of the following electron groups are on the nitrogen of methylamine (CH5N) ?
A) a double bond
B) a triple bond
C) a single electron
D) a lone pair of electrons
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The only interactions between two or more molecules of a nonpolar material such as methane (CH4) are _______ because these molecules do not have permanent dipoles.
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the structures are six electrons being shared between two atoms? 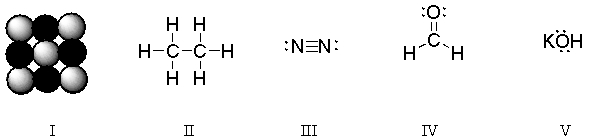
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ion that has more electrons than protons is a(n) ________.
A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these structures is a diatomic molecule? 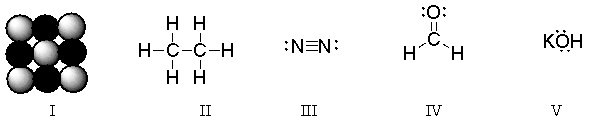
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following ionic formula is not valid. Which of the following statements best describes what's wrong with this formula? AlCl2
A) It does not include both a nonmetal and a metal.
B) The ratio of cations to anions is not 1:1.
C) The anion is written first, followed by the cation.
D) This compound does not exist in nature.
E) The charges do not add up to zero.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis dot structure is the same molecule as 2-methylbutane, shown below in its ball-and-stick model?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements best describes the meaning of an expanded octet?
A) "Expanded octets" are molecules in which an element expands its valence shell to gain an octet of electrons.
B) "Expanded octets" is a term that describes atoms that have more than eight electrons in their valence shells.
C) "Expanded octets" refers to atoms that, when bonded, must be larger than normal.
D) "Expanded octets" is used to describe elements that do not normally form an octet when bonded and would therefore have to expand to form the octet.
E) "Expanded octets" is used to describe how atoms accept electrons to attain a full valence shell.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of Na2SO3, a preservative in many foods and drinks?
A) sodium sulfite
B) sodium sulfate
C) disodium sulfur trioxide
D) sodium sulfur trioxide
E) disodium trisulfite
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules exhibits the strongest intermolecular forces of attraction?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a valid ionic formula?
A) NaF
B) MgO
C) KCl2
D) CaF2
E) Na2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the most likely connectivity of the atoms in chloroethylene (C2H3Cl) ?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is the most electronegative?
A) nitrogen
B) carbon
C) oxygen
D) sulfur
E) selenium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds is nonpolar?
A) C-O
B) O-F
C) C-F
D) C-H
E) H-F
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 164
Related Exams