A) two
B) three
C) four
D) six
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
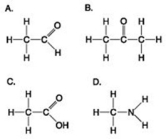 Which molecule has at least one carbon atom attached to three different chemical groups?
Which molecule has at least one carbon atom attached to three different chemical groups?
A) A
B) B
C) D
D) A and B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound contains hydroxyl groups as its predominant functional group. Therefore, this compound ________.
A) lacks an asymmetric carbon and is probably a fat or lipid
B) should dissolve in water
C) should dissolve in a nonpolar solvent
D) will not form hydrogen bonds with water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the term that correctly describes the relationship between these two sugar molecules:
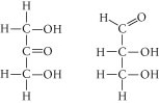
A) structural isomers
B) cis-trans isomers
C) enantiomers
D) isotopes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
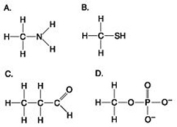 Which molecule shown is a thiol?
Which molecule shown is a thiol?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Amino acids are acids because they always possess ________ as the functional group?
A) amino
B) carbonyl
C) carboxyl
D) phosphate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which two functional groups are always found in amino acids?
A) carbonyl and amino groups
B) carboxyl and amino groups
C) amino and sulfhydryl groups
D) hydroxyl and carboxyl groups
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
C.
B.
 D.
Which functional group shown can pick up protons and raise the pH of the surrounding solution?
D.
Which functional group shown can pick up protons and raise the pH of the surrounding solution?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
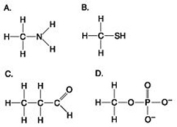 Which molecule can be a result of mercaptoethanol reduction of a disulfide bridge?
Which molecule can be a result of mercaptoethanol reduction of a disulfide bridge?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which action could produce a carbonyl group?
A) the replacement of the -OH of a carboxyl group with hydrogen
B) the addition of a thiol to a hydroxyl
C) the addition of a hydroxyl to a phosphate
D) the replacement of the nitrogen of an amine with oxygen
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that ________.
A) have identical chemical formulas but differ in the branching of their carbon skeletons
B) are mirror images of each other
C) differ in the location of their double bonds
D) differ in the arrangement of atoms around their double bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
C.
B. 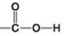 D.
Which of the functional groups shown helps stabilize proteins by forming covalent cross-links within or between protein molecules?
D.
Which of the functional groups shown helps stabilize proteins by forming covalent cross-links within or between protein molecules?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which chemical group is most likely to be responsible for an organic molecule behaving as a base?
A) hydroxyl
B) carbonyl
C) amino
D) phosphate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
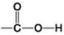
Each bond in carbon dioxide represents ________.
A) one resonating electron
B) a pair of shared electrons
C) two pairs of shared electrons
D) a pair of protons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
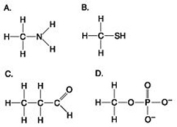 Which molecule shown above can contribute negative charge when positioned in a chain?
Which molecule shown above can contribute negative charge when positioned in a chain?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why are hydrocarbons insoluble in water?
A) The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C) They exhibit considerable molecular complexity and diversity.
D) They are less dense than water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true of carbon?
A) It forms only polar molecules.
B) It can form a maximum of three covalent bonds with other elements.
C) It is highly electronegative.
D) It can form both polar and nonpolar bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons does one atom of carbon share to complete its valence shell?
A) 2
B) 3
C) 4
D) 8
Correct Answer

verified
Correct Answer
verified
Showing 41 - 58 of 58
Related Exams