A) simple organic compounds can be synthesized in the laboratory from inorganic precursors, but complex organic compounds like carbohydrates and proteins can be synthesized only by living organisms
B) a life force ultimately controls the activities of living organisms, and this life force cannot be studied by physical or chemical methods
C) living organisms are composed of the same elements present in nonliving things, plus a few special trace elements found only in living organisms or their products
D) living organisms can be understood in terms of the same physical and chemical laws that can be used to explain all natural phenomena
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
C.
B. 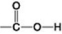 D.
Which of the functional groups shown is present in ethanol but not in ethane?
D.
Which of the functional groups shown is present in ethanol but not in ethane?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figure to answer the question.
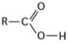 What is the name of the functional group shown in the figure?
What is the name of the functional group shown in the figure?
A) carbonyl
B) ketone
C) aldehyde
D) carboxyl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
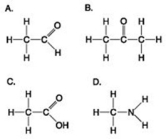 Which molecule shown contains a carboxyl group?
Which molecule shown contains a carboxyl group?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is carbon so important in biology?
A) It is a common element on Earth.
B) It has very little electronegativity, making it a good electron donor.
C) It bonds to only a few other elements.
D) It can form a variety of carbon skeletons and host functional groups.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
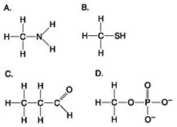 Which molecules shown contain a carbonyl group?
Which molecules shown contain a carbonyl group?
A) A and B
B) B and C
C) B, C, and D
D) C and D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figure to answer the question.
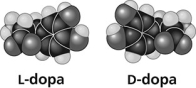 Thalidomide and L-dopa (see figure) are examples of pharmaceutical drugs that occur as enantiomers, or molecules that ________.
Thalidomide and L-dopa (see figure) are examples of pharmaceutical drugs that occur as enantiomers, or molecules that ________.
A) have identical three-dimensional shapes
B) are mirror images of one another
C) are mirror images of one another and have the same biological activity
D) are cis-trans isomers
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is polar? C₃H₇OHC₂H₅COOH
A) C₃H₇OH and C₂H₅COOH are both polar molecules.
B) Neither C₂H₅COOH or C₃H₇OH is polar.
C) C₂H₅COOH is polar, but C₃H₇OH is not polar.
D) C₂H₅COOH is not polar, but C₃H₇OH is polar.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Organic chemistry is currently defined as
A) the study of compounds made only by living cells.
B) the study of carbon compounds.
C) the study of natural (as opposed to synthetic) compounds.
D) the study of hydrocarbons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the question.
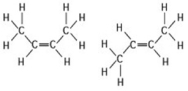 The two molecules shown in the figure are best described as ________.
The two molecules shown in the figure are best described as ________.
A) enantiomers
B) radioactive isotopes
C) structural isomers
D) cis-trans isomers
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
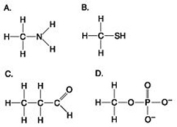 Which molecule shown above contains a functional group that is a part of the molecule known as the "energy currency of living organisms"?
Which molecule shown above contains a functional group that is a part of the molecule known as the "energy currency of living organisms"?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Miller's classic experiment demonstrated that a discharge of sparks through a mixture of gases could result in the formation of a large variety of organic compounds. Miller did not use ________ as one of the gases in his experiment.
A) methane
B) oxygen
C) water
D) ammonia
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon atom is most likely to form what kind of bond(s) with other atoms?
A) ionic
B) hydrogen
C) covalent
D) ionic bonds, covalent bonds, and hydrogen bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the question.
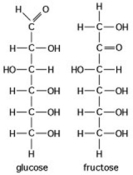 The figure shows the structures of glucose and fructose. These two molecules are ________.
The figure shows the structures of glucose and fructose. These two molecules are ________.
A) isotopes
B) enantiomers
C) cis-trans isomers
D) structural isomers
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the functional groups is not reactive but serves as a recognizable tag on the DNA molecule and alter the expression of genes in the cells.
A) amino
B) methyl
C) carboxyl
D) hydroxyl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complexity and variety of organic molecules is due to ________.
A) the chemical versatility of carbon atoms
B) the variety of rare elements in organic molecules
C) the diverse bonding patterns of nitrogen
D) their interaction with water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some carbon skeletons have different numbers and locations of double bonds to ________.
A) add molecular complexity and diversity that characterize living matter
B) be more flexible that makes the molecule stronger
C) stay in its liquid state
D) increase its solubility in water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Stanley Miller's 1953 experiments supported the hypothesis that ________.
A) life on Earth arose from simple inorganic molecules
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth
C) life on Earth arose from simple organic molecules, with energy from lightning and volcanoes
D) the conditions on early Earth were conducive to the origin of life
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon atom has 6 electrons however, its valency is 4. This is because the carbon atom ________.
A) donates its 2 electrons to another atom
B) shares its 2 electrons and bonds with another atom
C) has 4 electrons in its first shell and 2 in the second shell
D) has only 2 electrons in its first shell and 4 in the second shell
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Organic molecules with only hydrogens and five carbon atoms cannot ________.
A) have a branching carbon skeleton
B) have different combinations of double bonds between carbon atoms
C) have different positions of double bonds between carbon atoms
D) form enantiomers
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 58
Related Exams