A) dilithium carbon trioxide
B) lithium carbide
C) lithium carboxide
D) lithium carbonate
E) lithium tricarbonate
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What is the formula of the sulfate ion?
A) SO4-
B) SO3-
C) SO42-
D) SO32-
E) SO22-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the two principal classes of bonding called?
A) ionic bonding and nuclear bonding
B) covalent bonding and hydrogen bonding
C) hydrogen bonding and ionic bonding
D) polar bonding and ionic bonding
E) ionic bonding and covalent bonding
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula of the ionic compound sodium carbonate?
A) NaCO2
B) Na2CO2
C) Na2CO3
D) Na(CO3) 2
E) Na3CO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What kind of bonding exists in substances that consist of discrete molecules?
A) ionic
B) covalent
C) hydrogen bonding
D) intermolecular
E) polar
Correct Answer

verified
Correct Answer
verified
True/False
As a rule, a polar substance will be a good solvent for nonpolar solutes, and vice versa.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following Lewis structures has a possible resonance structure? 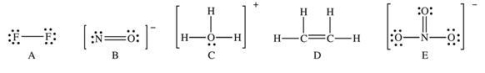
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of Fe2(SO4) 3 in the Stock system?
A) iron monosulfuric acid
B) iron(II) sulfate
C) iron(III) sulfate
D) iron trisulfate
E) iron(II) trisulfate
Correct Answer

verified
Correct Answer
verified
True/False
The name of SnO2 is tin(II) oxide.
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
According to the VSEPR theory, what is the geometry (shape) around the phosphorus atom in the Lewis structure shown below? 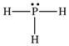
A) linear
B) bent (angular)
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is wrong with the Lewis structure shown for sulfur trioxide, SO3? 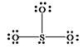
A) Oxygen, not sulfur, should be the central atom.
B) Sulfur should have 10 electrons around it instead of 8, to show its expanded octet.
C) The structure shows 26 valence electrons, but there should only be 24.
D) There are too many bonding electrons shown.
E) The structure should show each oxygen atom with a double bond to the sulfur atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the proper name for N2O5?
A) nitrogen(IV) oxide
B) dinitrogen pentoxide
C) nitrogen oxide
D) nitrate
E) nitrite
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the name of Fe2+ in the Stock system?
A) iron 2 ion
B) iron(II) ion
C) ferric(II) ion
D) ferrous 2+ ion
E) iron cation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of CuF2 in the Stock system?
A) copper(I) fluoride
B) copper(II) fluorate
C) copper difluoride
D) copper fluoride
E) copper(II) fluoride
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ionic compound iron(II) sulfate is used in iron-containing supplements.What does the (II) in the name of this compound specifically indicate?
A) There are two iron ions in this compound.
B) The charge on the iron ion is +2.
C) The charge on the sulfate ion is −2.
D) Iron has two valence electrons.
E) There are two sulfate ions in this compound.
Correct Answer

verified
Correct Answer
verified
True/False
The common name of iron(III) chloride is ferric chloride.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the Lewis symbol of the fluoride ion?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
True/False
In determining properties such as solubility, melting point, and boiling point, intramolecular forces are more important than intermolecular forces.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many total valence electrons are in SO42- ?
A) 2
B) 64
C) 32
D) 12
E) 16
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the ion whose formula is NO3-?
A) nitrogen trioxide ion
B) nitrate ion
C) ammonium ion
D) sodium oxide ion
E) nitride ion
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 91
Related Exams