A) concentration of reactants
B) concentration of the products
C) temperature
D) catalyst
E) size of reaction vessel
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction A(g) → 2B(g) , Keq = 4.5 × 105.Which statement is TRUE for the system at equilibrium?
A) [A] >> [B]
B) There is twice as much B as there is A in the reaction vessel.
C) There is more than 100 times more B than there is A in the reaction vessel.
D) There is twice as much A as there is B in the reaction vessel.
E) Changing the temperature will have no affect on the equilibrium.
Correct Answer

verified
Correct Answer
verified
True/False
A reaction that leads to a decrease in the enthalpy of the system is always spontaneous.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many small calories are equivalent to one nutritional Calorie?
A) 10
B) 100
C) 1,000
D) 10,000
E) 100,000
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of a chemical reaction increases with an increase in concentration of one or more of the reactants.This is best explained by which of the following statements?
A) The increased concentration of the reactants increases the temperature of the molecules.
B) The increased concentration of the reactants increases the activation energy of the reaction.
C) The increased concentration of the reactants increases the speed of the molecules.
D) The increased concentration of the reactants increases the frequency of effective collisions.
E) The increased concentration of the reactants decreases the activation energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In kinetics, the order of a reaction
A) is the inverse of the entropy of the system.
B) must be measured experimentally.
C) can be deduced from the balanced equation for the reaction.
D) depends on the rate constant.
E) depends on the concentrations of reactants.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement concerning energy changes in chemical reactions is FALSE?
A) The breaking of bonds requires energy, and the formation of bonds releases energy.
B) Activation energy is the minimum amount of energy required to initiate a chemical reaction.
C) A reaction is exothermic if the products have a lower energy than the reactants.
D) A reaction is endothermic if the reactants have a lower energy than the products.
E) The overall change in enthalpy (∆H) for a reaction is determined by the difference in energy between the products and the activated complex.
Correct Answer

verified
Correct Answer
verified
True/False
A reaction that leads to an increase in the entropy of the system is always spontaneous.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pairs of substances has the substance with the highest entropy listed first?
A) Ni(s) , Br2(l)
B) Hg(l) , He(g)
C) Br2(l) , H2O(g)
D) Ne(g) , H2O(l)
E) Ni(s) , Br2(l) and Ne(g) , H2O(l) are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement concerning a reversible reaction at equilibrium is FALSE?
A) The concentration of the products is equal to the concentration of the reactants.
B) The forward and reverse reaction rates are equal.
C) There is no further change in the amount of reactants and products.
D) A stress to the system would cause the system to shift in the direction that best relieves the stress.
E) All of the statements are true for a reversible reaction at equilibrium.
Correct Answer

verified
Correct Answer
verified
True/False
Changing the temperature alters the value of the equilibrium constant for a reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning the reaction below is FALSE? 2HgO(s) → 2Hg(l) + O2(g) ∆H = 182 kJ
A) There is an increase in entropy in this reaction.
B) This reaction is endothermic.
C) 182 kJ of energy are required for every two moles of HgO that react.
D) The energy of the reactants is greater than the energy of the products.
E) The system absorbs energy from the surroundings in this reaction.
Correct Answer

verified
Correct Answer
verified
True/False
A catalyst increases the equilibrium constant for a reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbohydrate sample weighing 0.235 g was found to have a fuel value of 3.84 kJ.What is the fuel value of one gram of this carbohydrate, in nutritional Calories?
A) 3,910 Cal
B) 0.535 Cal
C) 16.3 Cal
D) 643 Cal
E) 3.91 Cal
Correct Answer

verified
Correct Answer
verified
Essay
Consider the reversible reaction:
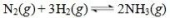 What is the correct expression for the equilibrium constant, Keq, for this reaction?
What is the correct expression for the equilibrium constant, Keq, for this reaction?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ethylene glycol has a specific heat of 0.578 cal/(g•°C) .If 23.2 g of ethylene glycol absorbs 75.6 cal of heat energy, what will the temperature increase be?
A) 0.177°C
B) 1.88°C
C) 5.64°C
D) 1.01 × 103 °C
E) 3.03 × 103 °C
Correct Answer

verified
Correct Answer
verified
True/False
Endothermic reactions are never spontaneous.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a sample of aqueous hydrochloric acid was neutralized with aqueous sodium hydroxide in a calorimeter, the temperature of 100.0 g of water surrounding the reaction increased from 25.0°C to 31.5°C.If the specific heat of water is 1.00 cal/(g•°C) , calculate the quantity of energy in calories involved in this neutralization reaction.
A) 1000 cal
B) 100.0 cal
C) 6.50 cal
D) 1250 cal
E) 650 cal
Correct Answer

verified
Correct Answer
verified
True/False
The equilibrium constant expression for a reaction can be written if the balanced equation is known.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One effect of a catalyst being added to a reaction mixture is
A) to increase the equilibrium constant for the reaction.
B) to slow down the rate of the reverse reaction.
C) to raise the temperature of the mixture.
D) to provide a new pathway for the reaction.
E) None of the choices are correct.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 61
Related Exams