A) 38.0
B) 0.278
C) 13.2
D) 2.21
E) 42.0
Correct Answer

verified
Correct Answer
verified
True/False
Heterogeneous catalysts have different phases from reactants.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN) : CH3NC (g) → CH3CN (g) At the start of an experiment, there are 0.200 mol of reactant and 0 mol of product in the reaction vessel. After 25 min, 0.108 mol of reactant (CH3NC) remain. There are _ _ mol of product (CH3CN) in the reaction vessel.
A) 0.200
B) 0.308
C) 0.540
D) 0.022
E) 0.092
Correct Answer

verified
Correct Answer
verified
True/False
The concentration of reactants or products at any time during the reaction can be calculated from the integrated rate law.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A catalyst can increase the rate of a reaction .
A) by providing an alternative pathway with a lower activation energy
B) by lowering the overall activation energy (Ea) of the reaction
C) by lowering the activation energy of the reverse reaction
D) by changing the value of the frequency factor (A)
E) All of these are ways that a catalyst might act to increase the rate of reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The enzyme nitrogenase converts _ _ into _.
A) nitrogen oxides, N2 and O2
B) nitroglycerine, nitric acid, and glycerine
C) ammonia, urea
D) CO and unburned hydrocarbons, H2O and CO2
E) nitrogen, ammonia
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction 2NO2 → 2NO + O2 Follows second- order kinetics. At 300°C, [NO2] drops from 0.0100- to 0.00650- M in 100 s. The rate constant for the reaction is _ M- 1s- 1.
A) 1.2
B) 0.096
C) 0.65
D) 0.54
E) 0.81
Correct Answer

verified
Correct Answer
verified
Short Answer
Reaction rate data obey an equation devised by _ .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: A - 2C The average rate of appearance of C is given by O[C]/Ot. Comparing the rate of appearance of C and the rate of disappearance of A, we get O[C]/Ot = × (O[A]/Ot) .
A) +1/2
B) - 1/2
C) +2
D) +1
E) - 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) →ClO3- (aq) + ClO2- (aq) + H2O (1) 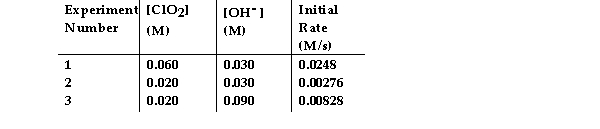 -What is the magnitude of the rate constant for the reaction?
-What is the magnitude of the rate constant for the reaction?
A) 4.6
B) 115
C) 230
D) 713
E) 1.15 × 104
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In general, as temperature goes up, reaction rate .
A) goes up if the reaction is endothermic
B) goes up if the reaction is exothermic
C) stays the same regardless of whether the reaction is exothermic or endothermic
D) goes up regardless of whether the reaction is exothermic or endothermic
E) stays the same if the reaction is first order
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of disappearance of HBr in the gas phase reaction 2HBr (g) → H2 (g) + Br2 (g) Is 0.190 M s- 1 at 150°C. The rate of reaction is M s- 1.
A) 2.63
B) 0.086
C) 0.0361
D) 0.095
E) 0.380
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The concentration of S2O82- remaining at 400 s is _ M.
A) +0.057
B) - 0.007
C) +0.015
D) +0.035
E) +0.045
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate constant for a second- order reaction is 0.13 M- 1s- 1. If the initial concentration of reactant is 0.26 mol/L, it takes s for the concentration to decrease to 0.13 mol/L.
A) 1.0
B) 0.017
C) 30
D) 4.4 × 10- 3
E) 0.50
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The kinetics of the reaction below were studied and it was determined that the reaction rate did not change when the concentration of B was tripled. The reaction is order in B. A + B → P
A) zero
B) first
C) second
D) third
E) one- half
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The graph shown below depicts the relationship between concentration and time for the following chemical reaction.
![The graph shown below depicts the relationship between concentration and time for the following chemical reaction. The slope of this line is equal to . A) ln[A]<sub>o</sub><sub> </sub> B) 1/k C) - k D) - 1/k E) k](https://d2lvgg3v3hfg70.cloudfront.net/TB1819/11ead62c_1f6c_7cc7_ac95_cb7f9ec7b75a_TB1819_00.jpg) The slope of this line is equal to .
The slope of this line is equal to .
A) ln[A]o
B) 1/k
C) - k
D) - 1/k
E) k
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The average rate of disappearance of I- between 400 s and 800 s is M/s.
A) 1.4 × 10- 5
B) 3.6 × 104
C) 5.8 × 10- 5
D) 2.6 × 10- 4
E) 2.8 × 10- 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g) → 4NO2 (g) + O2 (g) When the rate of formation of NO2 is 5.5 × 10- 4 M/s, the rate of decomposition of N2O5 is M/s)
A) 10.1 × 10- 4
B) 5.5 × 10- 4
C) 2.8 × 10- 4
D) 2.2 × 10- 3
E) 1.4 × 10- 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the units below, are appropriate for a first- order reaction rate constant.
A) s- 1
B) mol/L
C) L mol- 1 s- 1
D) M s- 1
E) .M- 1 s- 1
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 110
Related Exams