A) -1
B) +1
C) 0
D) +2
E) +3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the compound with covalent bonding.
A) Li2S
B) SrO
C) CaI2
D) C2Cl4
E) SrBr2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for PO43⁻.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the compound with the highest magnitude of lattice energy.
A) MgO
B) BaO
C) SrO
D) CaO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for SeO42⁻.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for SF4.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
The Lewis structure of BrO3- is as follows: 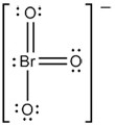 What is the formal charge on Br atom?
What is the formal charge on Br atom?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the bond below that is the weakest.
A) Na-Cl
B) I-I
C) C=N
D) Li-F
E) C=O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or compound below contains a polar covalent bond?
A) C2H4
B) ZnS
C) LiI
D) NCl3
E) AgCl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -Ca-O
A) strongest covalent bond
B) longest covalent bond
C) weakest ionic bond
D) metallic bond
E) highest melting point
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE?
A) An ionic bond is much stronger than most covalent bonds.
B) An ionic bond is formed through the sharing of electrons.
C) Ionic compounds at room temperature typically conduct electricity.
D) Once dissolved in water, ionic compounds rarely conduct electricity.
E) None of the above is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the bond below that is most polar.
A) H-I
B) H-Br
C) H-F
D) H-Cl
E) C-H
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represent the Lewis structure for Cl?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for NH4⁺.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -Sr-Sr
A) strongest covalent bond
B) longest covalent bond
C) weakest ionic bond
D) metallic bond
E) highest melting point
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for OCl2.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
The Lewis structure of acetate ion is as follows:
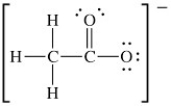 What is the formal charge on oxygen atom that contains three lone pairs of electrons?
What is the formal charge on oxygen atom that contains three lone pairs of electrons?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using periodic trends, place the following bonds in order of increasing ionic character. S-F Se-F O-F
A) Se-F < S-F < O-F
B) S-F < Se-F < O-F
C) O-F < Se-F < S-F
D) Se-F < O-F < S-F
E) O-F < S-F < Se-F
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the substance that conducts electricity.
A) NaCl dissolved in water
B) solid NaCl
C) water
D) solid sugar
E) sugar dissolved in water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the bond energies provided to estimate ΔrH° for the reaction below. PCl3(g) + Cl2(g) → PCl5(l) ΔrH° = ? Bond Bond Energy (kJ mol-1) Cl-Cl 243 P-Cl 331
A) -243 kJ mol-1
B) -419 kJ mol-1
C) -662 kJ mol-1
D) -67 kJ mol-1
E) -905 kJ mol-1
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 155
Related Exams