A) ![]() Na
Na
B) ![]() F
F
C) ![]() N
N
D) ![]() O
O
E) ![]() Ne
Ne
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What percentage of a radioactive substance remains after 7.00 half-lives have elapsed?
A) 0.391%
B) 0.78%
C) 1.56%
D) 3.12%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine how many neutrons are produced during the neutron-induced fission of 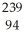 Pu to form
Pu to form 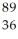 Kr and
Kr and 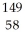 Ce.
Ce.
A) 2
B) 0
C) 3
D) 1
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write a nuclear equation for the alpha decay of 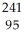 Am.
Am.
A) ![]() Am →
Am → ![]() He +
He + ![]() Np
Np
B) ![]() Am →
Am → ![]() He +
He + ![]() Bk
Bk
C) ![]() Am →
Am → ![]() e +
e + ![]() Cm
Cm
D) ![]()
E) ![]() Am →
Am → ![]() n +
n + ![]() Am
Am
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents what nuclear process? 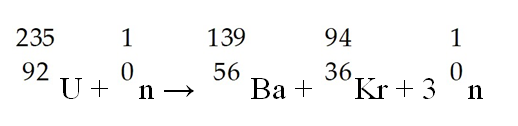
A) nuclear fission
B) nuclear fusion
C) electron capture
D) alpha decay
E) beta emission
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations shows the correct relationship between the half-life of a nuclide and the radioactive decay rate constant?
A) ln ![]() = -
= - ![]()
B) ![]() = - ln
= - ln ![]()
C) ![]() = 0.693 × k
= 0.693 × k
D) ![]() =
= ![]()
E) ![]() =
= ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Describe what is meant by the "valley of stability."
Correct Answer

verified
If a plot of number of neutrons vs. numb...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following nuclides are most likely to decay via beta decay?
A) I-126
B) Al-24
C) N-13
D) Cs-137
E) Na-20
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the positron emission of  O.
O.
A) ![]() C
C
B) ![]() F
F
C) ![]() N
N
D) ![]() N
N
E) ![]() C
C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents what nuclear process? \
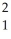 H +
H + 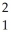 H →
H → 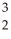 He +
He + 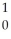 n
n
A) nuclear fusion
B) alpha emission
C) beta emission
D) nuclear fission
E) neutron capture
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -proton
A) ![]() e
e
B) ![]() p
p
C) ![]() He
He
D) ![]() g
g
E) ![]() n
n
F) ![]() e
e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents what nuclear process?
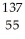 Cs +
Cs + 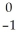 e →
e → 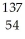 Xe
Xe
A) beta emission
B) positron emission
C) gamma emission
D) electron capture
E) alpha capture
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the beta decay of 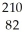 Pb.
Pb.
A) ![]() Pt
Pt
B) ![]() Tl
Tl
C) ![]() Hg
Hg
D) ![]() Bi
Bi
E) ![]() Pb
Pb
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents which nuclear process? 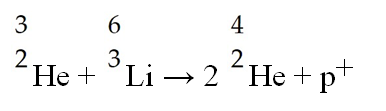
A) nuclear fission
B) nuclear fusion
C) electron capture
D) alpha decay
E) beta emission
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fluorine-18 undergoes positron emission with a half-life of 1.10 × 102 minutes. If a patient is given a 248 mg dose for a PET scan, how long will it take for the amount of fluorine-18 to drop to 83 mg? (Assume that none of the fluorine is excreted from the body.)
A) 99 minutes
B) 1.7 × 102 minutes
C) 1.3 × 102 minutes
D) 3.0 × 102 minutes
E) 2.1 × 102 minutes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclide As-76 has a half-life of 26.0 hours. If a sample of As-76 weighs 344 g, what mass of As-76 remains after 538 minutes?
A) 67.8 g
B) 271 g
C) 144 g
D) 437 g
E) 251 g
Correct Answer

verified
Correct Answer
verified
Showing 101 - 116 of 116
Related Exams