A) Transition metals
B) s-block elements
C) Main group nonmetals
D) Noble gases
E) Semiconductors
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below? 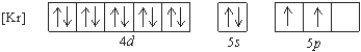
A) n = 1, ![]() = 1,
= 1, ![]() = -1,ms = +1/2
= -1,ms = +1/2
B) n = 4, ![]() = 2,
= 2, ![]() = -1,ms = -1/2
= -1,ms = -1/2
C) n = 5, ![]() = 2,
= 2, ![]() = -2,ms = +1/2
= -2,ms = +1/2
D) n = 5, ![]() = 0,
= 0, ![]() = 0,ms = -1/2
= 0,ms = -1/2
E) n = 5, ![]() = 1,
= 1, ![]() = -1,ms = +1/2
= -1,ms = +1/2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the following ground state electron configuration? 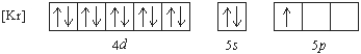
A) In
B) Y
C) Nb
D) Tl
E) Ga
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following orbital occupancy designations is incorrect?
A) 2s2
B) 3d6
C) 1s2
D) 4p3
E) 3d12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ions has the given ground state electron configuration? 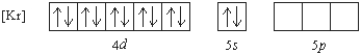
A) Cd2+
B) Sr2+
C) Zn2+
D) Sn2+
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A metal oxide forms when potassium reacts with oxygen.What is the most likely formula of this metal oxide?
A) KO
B) K2O
C) K2O3
D) KO2
E) KO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The change in energy for the following reaction is referred to as the ____ for boron. B(g) + e- → B-(g)
A) oxidation number
B) electron affinity
C) electronegativity energy
D) first ionization energy
E) second ionization energy
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atom has the ground state electronic configuration 1s22s22p63s23p64s23d3?
A) Ga
B) V
C) As
D) Nb
E) none
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many unpaired electrons are found in the ground state electron configuration of barium (ba) ?
A) 0
B) 1
C) 2
D) 3
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has the ground state electron configuration [Ar]3d104s1?
A) Cu
B) Zn
C) Ge
D) Ag
E) Cd
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following ground-state electron configurations are correct except
A) V: [Ar]4s24d3
B) K: [Ar]4s1
C) Sb: [Kr]4d105s25p3
D) Cr: [Ar]3d54s1
E) Te: [Kr]4d105s25p4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following orbital diagrams represents a paramagnetic atom? 1s 2s 2p
1) 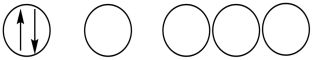 2.
2. 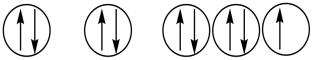 3.
3. 
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following atoms in order decreasing atomic radii: Be,Be,Be,B.
A) B > Be > Be > Be
B) Be > B > Be > Be
C) Be > Be > B > Be
D) Be > Be > Be > B
E) B > Be > Be > Be..
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements in its 1+ ionic state has the ground state electron configuration [Kr]4d10?
A) Ru
B) Au
C) Ag
D) In
E) Cd
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons can be described by the quantum numbers n= 3 and l= 2?
A) 14
B) 6
C) 2
D) 18
E) 10
Correct Answer

verified
E
Correct Answer
verified
Short Answer
________ rule states that the most stable arrangement of electrons is that which contains the maximum number of unpaired electrons,all with the same spin direction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ground-state electron configuration of a Ni2+ ion is 1s22s22p63s23p63d8 .Therefore,Ni2+ is
A) paramagnetic with two unpaired electrons.
B) diamagnetic.
C) paramagnetic with one unpaired electron.
D) paramagnetic with four unpaired electrons.
E) paramagnetic with five unpaired electrons.
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following electron configurations corresponds to the ground state of an atom of a transition element?
A) 1s22s22p1
B) 1s22s22p63s23p63d104s24p3
C) 1s22s22p63s23p63d14s2
D) 1s22s22p63s23p64s1
E) 1s22s22p63s23p4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A metal phosphide forms when potassium reacts with elemental phosphorus.What is the most likely formula of this metal phosphide?
A) KP
B) K3P
C) K2P3
D) K3P2
E) KP3
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the ground state electron configuration for Cr3+?
A) [Ar]
B) [Ar]3d74s2
C) [Ar]3d14s2
D) [Ar]3d24s1
E) [Ar]3d3
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 80
Related Exams