A) O2
B) CO2
C) H2O
D) H2
E) All have the same average kinetic energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen dioxide is a red-brown gas that is responsible for the color of photochemical smog. A sample of nitrogen dioxide has a volume of 28.6 L at 45.3°C and 89.9 kPa. What is its volume at STP?
A) 21.8 L
B) 27.6 L
C) 29.6 L
D) 37.6 L
E) 153 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask containing helium gas is connected to an open-ended mercury manometer. The open end is exposed to the atmosphere, where the prevailing pressure is 752 torr. The mercury level in the open arm is 26 mm above that in the arm connected to the flask of helium. What is the helium pressure, in torr?
A) -26 torr
B) 26 torr
C) 726 torr
D) 778 torr
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its Celsius temperature, other factors remaining constant? 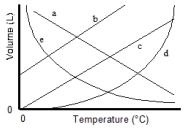
A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
True/False
According to the postulates of kinetic-molecular theory, the molecules of all gases at a given temperature have the same average kinetic energy.
Correct Answer

verified
Correct Answer
verified
True/False
For an ideal gas, a plot of PV/nRT versus P gives a straight line with a positive slope.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask with a volume of 3.16 L contains 9.33 grams of an unknown gas at 32.0°C and 1.00 atm. What is the molar mass of the gas?
A) 7.76 g/mol
B) 66.1 g/mol
C) 74.0 g/mol
D) 81.4 g/mol
E) 144 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"The rate of effusion of a gas is inversely proportional to the square root of its molar mass" is a\ statement of ______________________ Law.
A) Charles's
B) Graham's
C) Dalton's
D) Avogadro's
E) Boyle's
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Helium gas is being pumped into a rigid container at a constant temperature. As a result, the pressure of helium in the container is increasing. Select the one correct statement below.
A) As the pressure increases, helium atoms move faster, on average.
B) As the pressure increases, helium atoms move more slowly, on average.
C) As the pressure increases, the volume of the container must decrease.
D) As the pressure increases, helium atoms stay closer to the wall of the container, on average.
E) As the pressure increases, there are more collisions of helium atoms with the container wall.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of nitrogen gas at 298 K and 745 torr has a volume of 37.42 L. What volume will it occupy if the pressure is increased to 894 torr at constant temperature?
A) 22.3 L
B) 31.2 L
C) 44.9 L
D) 112 L
E) 380 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas mixture, with a total pressure of 300. torr, consists of equal masses of Ne (atomic weight 20.) and Ar (atomic weight 40.) . What is the partial pressure of Ar, in torr?
A) 75 torr
B) 100. torr
C) 150. torr
D) 200. torr
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen will behave most like an ideal gas
A) at high temperature and high pressure.
B) at high temperature and low pressure.
C) at low temperature and high pressure.
D) at low temperature and low pressure.
E) at intermediate (moderate) temperature and pressure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of propane, a component of LP gas, has a volume of 35.3 L at 315 K and 922 torr. What is its volume at STP?
A) 25.2 L
B) 30.6 L
C) 33.6 L
D) 37.1 L
E) 49.2 L
Correct Answer

verified
Correct Answer
verified
True/False
For real gases, PV > nRT, always.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that pressure has dimensions of force ÷ area; that force has dimensions of mass × acceleration; and that the S.I. unit of pressure is the pascal, what is 1 pascal in terms of S.I. base units?
A) 1 Pa = 1000 g/cm·s2
B) 1 Pa = 1 g/m·s2
C) 1 Pa = 10-3 kg·m/s2
D) 1 Pa = 1 kg·m/s2
E) 1 Pa = 1 kg/m·s2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Methane, CH4(g) , reacts with steam to give synthesis gas, a mixture of carbon monoxide and hydrogen, which is used as starting material for the synthesis of a number of organic and inorganic compounds. CH4(g) + H2O(g) → CO(g) + H2(g) [unbalanced] What mass of hydrogen is formed if 275 L of methane (measured at STP) is converted to synthesis gas?
A) 12.3 g
B) 24.7 g
C) 37.1 g
D) 49.4 g
E) 74.2 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the rms speed of carbon dioxide molecules at STP.
A) 12.4 m/s
B) 155 m/s
C) 393 m/s
D) 1.55 × 105 m/s
E) The answer can't be calculated without more data.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 97 of 97
Related Exams