A) 1s2,2s2,2p5
B) 1s2,2s2,2p2
C) 1s2,2s2,2p6
D) 1s2,2s2,2p4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a resonance structure of the compound below? 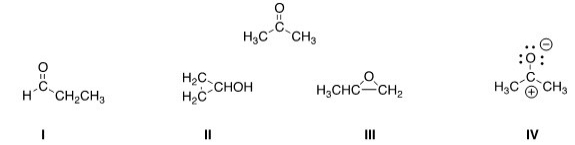
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes the typical number of bonds for carbon,nitrogen,and oxygen in most neutral organic molecules?
A) Carbon forms 4 covalent bonds,nitrogen forms 2 covalent bonds,and oxygen forms 3 covalent bonds.
B) Carbon forms 4 covalent bonds,nitrogen forms 3 covalent bonds,and oxygen forms 2 covalent bonds.
C) Carbon forms 4 covalent bonds,nitrogen forms 5 covalent bonds,and oxygen forms 2 covalent bonds.
D) Carbon forms 4 covalent bonds,nitrogen forms 5 covalent bonds,and oxygen forms 4 covalent bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate H-C-O bond angle in formaldehyde,H2CO?
A) 90°
B) 109.5°
C) 120°
D) 180°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-carbon s bonding molecular orbital of acetylene,C2H2?
A) Csp2 + Csp2
B) Csp + Csp
C) Csp3 + Csp3
D) C2p + C2p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate bond angle for the C-C-N bond in acetonitrile,CH3CN?
A) 90°
B) 109.5°
C) 120°
D) 180°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Avobenzone is an active ingredient in some common sunscreens.Which of the following is the correct molecular formula for avobenzone? 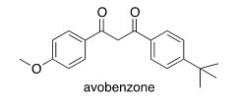
A) C22O22O3
B) C20H22O3
C) C21H23O3
D) C20H24O3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the appropriate conversion of (CH3) 4C to a skeletal structure? 
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has the smallest dipole moment?
A) CO2
B) HCl
C) H2O
D) NH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate C-C-C bond angle in propene,CH3CH = CH2?
A) 90°
B) 109.5°
C) 120°
D) 180°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pair does not represent resonance structures? 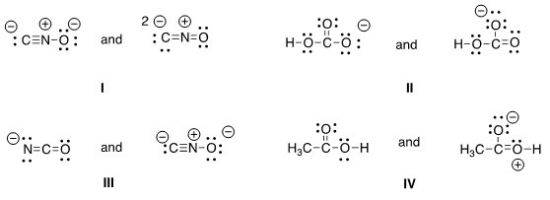
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules does not have a net dipole moment of zero?
A) CCl4
B) BF3
C) CO2
D) NH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following ions does carbon have a formal charge? 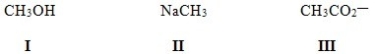
A) I
B) II
C) III
D) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many constitutional isomers are there for a molecule having the molecular formula C3H8O?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization for each of the indicated atoms in the following compound? 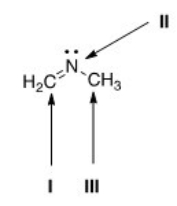
A) I = sp2; II = sp2; III = sp2.
B) I = sp2; II = sp3; III = sp3.
C) I = sp; II = sp2; III = sp3.
D) I = sp2; II = sp2; III = sp3.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the condensed formula of the compound below? 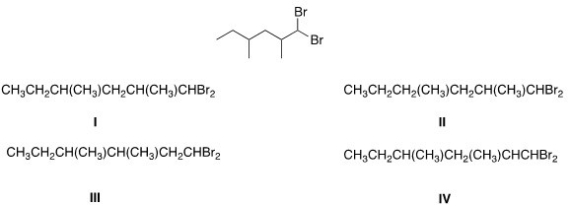
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the C-H s bonding molecular orbitals of ethane,CH3CH3?
A) Csp2 + H1s
B) Csp3 + H1s
C) C2p + H1s
D) Csp + H1s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following ions does carbon have a formal charge? 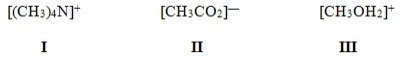
A) I
B) II
C) III
D) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which structure is the hybridization incorrect? 
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about bonding is true?
A) Covalent bonds result from the transfer of electrons from one element to another.
B) Ionic bonds result from the transfer of electrons from a metal to a non-metal.
C) Ionic bonds result from the sharing of electrons between two non-metals.
D) Covalent bonds result from the sharing of electrons between two metals.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 77
Related Exams