A) sp3 hybridized
B) sp2 hybridized
C) sp hybridized
D) not hybridized
Correct Answer

verified
B
Correct Answer
verified
Short Answer
Give the ground-state electron configuration for magnesium (atomic number 12).
Correct Answer

verified
Correct Answer
verified
Short Answer
Exhibit 1-3
Determine the hybridization for the indicated atoms in each structure below. 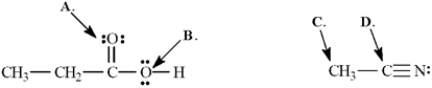 -Refer to Exhibit 1-3.The hybridization of this carbon atom (C) is ______.
-Refer to Exhibit 1-3.The hybridization of this carbon atom (C) is ______.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If all the missing bonds in the following structure are sigma bonds to hydrogen atoms,how many hydrogen atoms are missing from this structure? Atoms other than carbon are labeled. 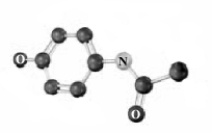
A) 7
B) 10
C) 12
D) 14
E) None of these is the correct number.
Correct Answer

verified
E
Correct Answer
verified
Short Answer
Give the ground-state electron configuration for fluorine (atomic number 9).
Correct Answer

verified
1s22s22px2 2py2 ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw the orbital diagram showing the ground-state electron configuration of sulfur.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hybridization of the atomic orbitals shown would result in: 


A) sp3 hybridization
B) sp2 hybridization
C) sp hybridization
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents a hybrid orbital?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of hybridization is exhibited by carbon in the following substance?: 
A) sp3 hybridization
B) sp2 hybridization
C) sp hybridization
Correct Answer

verified
Correct Answer
verified
Short Answer
Exhibit 1-3
Determine the hybridization for the indicated atoms in each structure below. 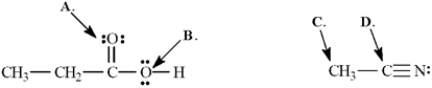 -Refer to Exhibit 1-3.The hybridization of this oxygen atom (B) is ______.
-Refer to Exhibit 1-3.The hybridization of this oxygen atom (B) is ______.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of hybridization is exhibited by carbon in the following substance?: 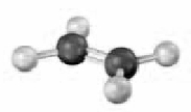
A) sp3 hybridization
B) sp2 hybridization
C) sp hybridization
Correct Answer

verified
Correct Answer
verified
Essay
Draw an orbital picture for acetylene,C2H2.Clearly label each bond type and indicate the type of orbitals involved in each bond.
Correct Answer

verified
11eab917_152b_cdfd_99e6_b723168f76f5_TB4944_00
Correct Answer
verified
Essay
There are two substances with the molecular formula C2H7N.Draw them and describe how they differ.
Correct Answer

verified

 The two structures differ in the numbe...
The two structures differ in the numbe...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
How many electrons does silicon have in its valence shell?
Correct Answer

verified
Correct Answer
verified
Essay
Convert the following structure to a skeletal drawing and give its molecular formula. 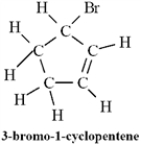
Correct Answer

verified
 Molecular...
Molecular...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
The original question was combined with #15.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.
Correct Answer

Answered by ExamLex AI
The input question does not provide a sp...View Answer
Show Answer
Correct Answer
Answered by ExamLex AI
View Answer
Essay
Convert the following molecular model into a condensed structure and a skeletal structure. 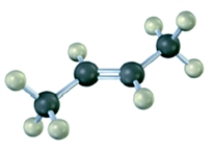
Correct Answer

verified
 or more s...
or more s...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Convert the following molecular model into a condensed structure and a skeletal structure. 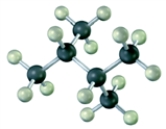
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Overlap of the two atomic orbitals shown could result in a: 

A) σ bond
B) π bond
C) σ or π depending on the direction of the overlap.
Correct Answer

verified
Correct Answer
verified
Short Answer
Give the ground-state electron configuration for carbon (atomic number 6).
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 29
Related Exams