Correct Answer

verified
11eab5e1_c8ac_5e86_9bae_1f7605c18b7c_TB6199_00
Correct Answer
verified
Essay
Give the structure of the alkene which would yield the following products upon ozonolysis-reduction. CH3CH2CH2CH2CHO + CH2O
Correct Answer

verified
CH3CH2CH2CH2CH 11eab5e1_c8b4_c358_9bae_8d6a88e2781a_TB6199_00 CH2
Correct Answer
verified
Essay
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 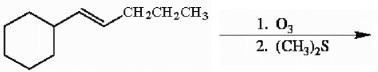
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provide the reagents necessary to complete the following transformation. 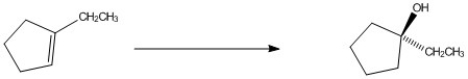
A) 1. BH3∙THF 2. H2O2, HO-
B) H2O, H2SO4
C) OsO4, H2O2
D) CH3CO3H
E) 1. CH3CO3H 2. H+, H2O
Correct Answer

verified
Correct Answer
verified
Essay
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 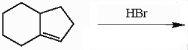
Correct Answer

verified
Correct Answer
verified
Essay
Draw the major regioisomeric product generated in the reaction below. 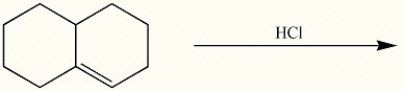
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provide the reagents necessary to complete the following transformation. 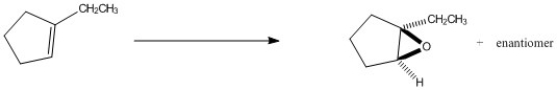
A) 1. BH3∙THF 2. H2O2, HO-
B) H2O, H2SO4
C) OsO4, H2O2
D) CH3CO3H
E) 1. CH3CO3H 2. H+, H2O
Correct Answer

verified
Correct Answer
verified
Essay
Give the structure of the alkene which would yield the following products upon ozonolysis-reduction. CH3COCH3 + CH3CH2CHO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following steps would successfully complete the following reaction? 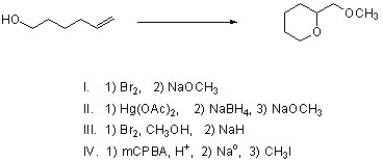
A) I only
B) II & III
C) I & IV
D) I, II, & IV
Correct Answer

verified
Correct Answer
verified
Essay
Provide the reagents necessary to complete the following transformation. 
Correct Answer

verified
1) Br2, hν
2) H2O, Δ
o...View Answer
Show Answer
Correct Answer
verified
2) H2O, Δ
o...
View Answer
Multiple Choice
When propylene reacts with hydrogen bromide in the presence of a peroxide initiator, which of the following structures are formed during the mechanism?
A) ![]()
B) ![]()
C) ![]()
D) H∙
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product of the following reaction? 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unknown compound with empirical formula C3H5 was treated with Br2/CCl4. The bromine solution went from orangish/red to clear immediately at room temperature. Upon treatment with O3 followed by work-up with dimethylsulfide the following products were identified. From the information provided what is/are the most likely structure(s) for this unknown compound. 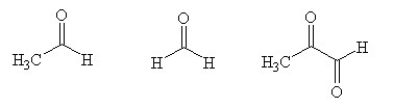
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) both A and D
Correct Answer

verified
Correct Answer
verified
Essay
Using any alkene as your starting material, how could you make the alkyl halide shown? 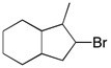
Correct Answer

verified
Correct Answer
verified
Essay
Consider how the I-Cl bond is polarized and predict the product which results when this mixed halogen adds to 1-methylcyclohexene.
Correct Answer

verified
Correct Answer
verified
Essay
Provide the major organic product of the reaction below. 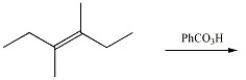
Correct Answer

verified
Correct Answer
verified
Essay
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 
Correct Answer

verified
11eab5e1_c8a9_c657_9bae_dd6c08283ff4_TB6199_00
Correct Answer
verified
Multiple Choice
Treatment of cyclopentene with peroxybenzoic acid ________.
A) results in oxidative cleavage of the ring to produce an acyclic compound
B) yields a meso epoxide
C) yields an equimolar mixture of enantiomeric epoxides
D) gives the same product as treatment of cyclopentene with OsO4
E) none of the above
Correct Answer

verified
Correct Answer
verified
Essay
Provide a detailed, step-by-step mechanism for the reaction shown below. 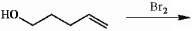
Correct Answer

verified
Correct Answer
verified
Short Answer
The mechanism for the acid-catalyzed hydration of alkenes is simply the reverse of the mechanism by which alcohols are dehydrated using concentrated acid. This is an illustration of the principle of ________.
Correct Answer

verified
microscopi...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 1 - 20 of 134
Related Exams